AUCTORES
Globalize your Research
Review Article | DOI: https://doi.org/10.31579/2639-4162/258
1 Department of Neurology, Hospital “Sant’Antonio Abate” Trapani, Italy.
2 Retired Professor of Human Anatomy. University of Palermo. Palermo, Italy.
3 Department of Medicine and Surgery of Acceptance and Emergency. Emergency Room (MCAU) of the Villa Sofia-Cervello Hospital in Palermo, Italy.
*Corresponding Author: Farina Elvira, Retired Professor of Human Anatomy. University of Palermo. Palermo, Italy.
Citation: Lipari Alessio, Farina Elvira, Lipari Luana (2025), Somatotopy of the Gracile, Cuneate, External Cuneate Nuclei, Sensory Pathway (Medial Lemniscus, Anterolateral System or Spinal Lemniscus) and Motor Pathway (Corticospinal and Corticobulbar Tracts), J. General Medicine and Clinical Practice, 8(4); DOI:10.31579/2639-4162/258
Copyright: © 2025, Farina Elvira. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 04 March 2025 | Accepted: 21 March 2025 | Published: 31 March 2025
Keywords: somatotopy; medial lemniscus; anterolateral system or spinal lemniscus; corticospinal tract; corticobulbar tract
Our previous studies concern the somatotopy of the spinal cord, the oculomotor and trigeminal complexes, the facial nuclei, thus in this review we extend the study to the somatotopy of the nuclei of the origin of the sensory pathways (medial lemniscus, anterolateral system or spinal lemniscus), the origin and course of the motor pathways (corticospinal an corticobulbar tracts) The study shows that lesions of sensory and motor tracts induce clinical aspects related to the level at which the lesion occurs. The knowledge of the origin and pathways is important to understand some neurological clinical and surgical aspects.
The knowledge of somatotopic location of the sensory and motor pathways is important for both neurological medical clinics and neurological surgery. It is useful and important to analyze both origin and the course of the sensory pathway (medial lemniscus and anterolateral system) since lesions of these sensory tracts induce different clinical effects and also lesions of the voluntary motor pathway (the pyramidal system) induce different motor consequence in relation to the level at which the lesion occurs. The pathway motor, pyramidal tract, has the main motor function, also, plays a sensory function modulating the transmission of stimuli from the spinal cord and, also, both pyramidal subsystem corticobulbar and corticospinal tracts modulate the processing and integration of the ascending somatosensory information generated by the movement itself.
Medial Lemniscus
The medial lemniscus origins by three nuclei: gracile, cuneate, external cuneate that typically form the dorsal column nuclei.
Nucleus gracilis and nucleus cuneatus
The nucleus gracilis and the nucleus cuneatus are two sensory nuclei of the brainstem located in the medulla oblongata postero-medially and postero-laterally respectively; the nucleus gracilis is capped dorsally and laterally by fibers of the fasciculus gracilis. These two nuclei form the two tubercles gracilis and cuneatus on the dorsal surface of the medulla oblongata,
The nuclei gracilis and cuneatus contain the second-order neurons which synapse with the fibers of the fasciculus gracilis or tract of Goll and fasciculus cuneatus or tract di Burdach which run medially and laterally respectively in the ipsilateral dorsal column of the spinal cord and are constituted of the fibers originating from the cell bodies, primary or first-order, located in the dorsal root ganglion and progressively increasing at higher levels their number.
The gracile fasciculus carries sensory input from level of the T6 vertebra and from the vertebrae caudal to it; the gracile fasciculus ascends and ends in the gracile nucleus which forms the gracile tubercleof dorsal surface of the medulla oblongata.
The cuneate fasciculus carries information from level T6 and from the rostral vertebrae to it: the cuneate fasciculus ascends and ends in the cuneate nucleus in the medulla oblongata which forms the cuneate tubercle. At levels the caudal part of the olivary complex the entire gracile fasciculus and most of cuneate fasciculus have replaced by their respective nuclei.
The gracile and cuneate nuclei (1) convey the information from the peripheral receptors and the discriminative touch and conscious proprioceptive information. Both nuclei have two regions: the superior regions with a reticular organization with multipolar small and large neurons which receive fibers originated from neurons of the gray matter of the spinal cord of all levels; the inferior regions constituted of clusters of the large neurons with short and branching dendrites.
In both nuclei the disposition of the afferent fibers is in relation to level of the origin of the dorsal spinal roots, so, the lower limbs are represented medially, the trunk anteriorly and the digits of the hand posteriorly and, further, in relation to the modality so, the inferior sectors respond to cutaneous low threshold stimuli, while the reticular sectors superior respond to stimuli that innervate the other receptors cutaneous and proprioceptive, articulate and muscular stimuli.
Gracile nucleus
Gracile nucleus is distinct in three regions:
1) Region reticular (region rostral to the obex) with a loose organization of cells;
2) Region “cell nest” (region caudal to the obex) with cell clusters;
3) Region caudal region with scattered cells.
The fibers of the gracile fascicle originate in dorsal root ganglion from the vertebra T6 and below vertebrae, run medially in the dorsal column of spinal cord and project somatotopically to the gracile nucleus.
1) roots of the lumbar enlargement project to irregular shaped areas in the central core of the nucleus,
2) lower thoracic and upper lumbar roots project in the serial fashion to narrow, oblique laminae lateral to the core region,
3) sacral and coccygeal dorsal roots project in serial fashion to crescent-shaped laminae in dorsomedial parts of the nucleus.
According to Hand (2) in cat the lumbosacral dorsal root fibers exhibit a somatotopic lamination chiefly in the “chest nest” region, while terminations in the reticular region are diffuse with intersegmental overlaop.
Cuneate nucleus
Cuneate nucleus (CN) firstly appears at mid-levels of the decussation of pyramids, has a cylinder-shaped structure that lies bilaterally in the brainstem and extends nearly 4mm in the rostrocaudal direction and extends to the rostral pole of the area postrema. The forelimb is represented along the rostrocaudal extent.
The dorsal areas of cuneate nucleus have round cells clusters and receive the afferent fibers from the distal parts of the body;
the basal areas of cuneate nucleus have triangular and multipolar cells and receive afferent fibers primarily from the proximal parts of the limb and trunk.
In the monkey (3) the cuneate nucleus receives: 1) root fibers from C1-T1 terminating in both exclusive and overlapping zones; 2) root fibers from C5-C8 terminating in a central core region about which other dorsal root fibers terminate in oblique serial laminae; 3) rostral root fibers (C1-C4) terminating in ventrolateral regions; 4) caudal dorsal root fibers (T1-T7) terminating in dorsomedial regions (4). The cuneate nucleus is subdivided in four zones: intermediate or main zone containing a “pars rotunda” and a “pars triangularis” situated more laterally. The pars rotunda is constituted of the elongated neurons disposed longitudinally which receive the afferents from the upper limb with a somatotopic distribution;
reticular superior pole and reticular inferior pole which receive modulatory facilitating or inhibiting fibers from the sensitive cerebral cortex and the reticular formation with a diffuse distribution.
The cuneate nucleus in monkey (5) receives the terminations from each digit formed an elongated column that was densely labelled in the central pars rotunda and sparsely labelled in both the rostral and caudal reticular poles. In particular the digits 1-5 were represented, in order from lateral to medial, within the “pars rotunda”. Inputs from the digit tips terminated ventral to inputs from the proximal digits. The afferents from the dorsal skin of the digits terminated in an even more dorsal position, while the most dorsal portion of pars rotunda is related to the glabrous and dorsal hand. Fibers from the thumb or digit 1 project lateral (and ventral) to those from digit 2, and projections from digit 3 are medial to those from 2. Each digital projection field is closely adjacent to that from the adjacent digit. Few fibers extend to the rostral CN. Projection fields of homologous digits are quite symmetrical on the two sides. The digit representations are topographically organized with the distal digit surface represented laterally and the proximal digit surface represented medially. The digit and palmar pads are also represented by barrelettes located on the medial side of the cuneate nucleus. In contrast, the dorsal digit surfaces are represented dorsally and the dorsal hand is represented directly beneath the cuneate fasciculus, in a region devoid of barrelettes. The forelimb representation is bordered on the medial side by representation of trunk and hindlimb, and on the lateral side by representation of shoulder, ear, and head. Recently the human cuneate nucleus (Cu) (6) has been subdivided in discrete subregions by neurochemical and histological features of sensory nuclei, so, a plexiform network of intense SP immunoreactivity is showed in the territory of the human Cu nucleus and cuneate fascicle. The SP- immunoreactive areas begin to appear at the level of the caudal reticular pole of the main Cu, continue rostrally, placed along the dorsal border of the main Cu evidencing their implication in the neurotransmission of protopathic stimuli, including pain,
The accessory cuneate nucleus or external cuneate nucleus (ECN) is lateral to the cuneatus nucleus and is constituted of large neurons (likely the nucleus thoracicus posterior of the spinal cord). The accessory nucleus receives the lateral fibers of the fascicle cuneatus conveying proprioceptive information of the upper limb that enter the spinal cord at cervical myelomeres. The axons of the accessory cuneate nucleus form the posterior external arcuate fibers which go to the spinocerebellum via ipsilateral cerebellar inferior peduncle.
The external cuneate nucleus ECN (7) in cat contains neurons (99,2%) organized in a somatotopic manner. Thus, the neurons of the distal occipitoscapularis muscles, (OOC), project to medial parts and neurons of the proximal the biventer cervicis muscle (BIV) project to more lateral parts of the nucleus. Forty-three of the 143 neurons in the ECN (30.1%) were activated antidromically to electrical stimulation of the ipsilateral inferior peduncle and anterior lobules IV and V of the cerebellum. In the thalamus the responses from nuchal muscle afferent fibers were recorded in a very narrow region of nucleus ventralis posterolateralis (VPL) which is situated dorsolaterally to the forelimb muscle afferent (deep radial nerve) projection area. In the cortex the evoked potentials from these nerve stimulations were observed in the anterior suprasylvian gyrus, areas 2 or 5, which we regarded as a transitional area between the second somatosensory and association areas, and the postcruciate dimple (PCD) or area 3a.
The external cuneate nucleus ECN in rat (8) receives proprioceptive inputs from forelimb and neck muscles and projects terminals in the ventromedial part of the VPL (VPLvm), but not in the rostrodorsal shell of the VPL of thalamus. Thus, these findings indicate that proprioception from forelimb/neck muscle spindles and jaw-closing muscle spindles (JCMSs) is somatotopically transmitted to the ventromedial floor of the ventrobasal thalamic complex, but not rosrodorsal shell.
Medial Lemniscus (Bulbothalamic Tract)
Medial lemniscus or bulbothalamic tract is a nerve tract which conveys sensory information related to discriminative touch and conscious proprioception from the body to cerebral cortex, begins in the two nuclei cuneate and gracile and ends in the ventral posterolateral nucleus of the thalamus.
Medial lemniscus or bulbothalamic tract consists of the axons or fibers of the neurons of the gracile and cuneatus nuclei which synapse with the fibers of the gracile and cuneate fasciculi located in the posterior funiculus or column of spinal cord (Fig. 1A) where the gracile fasciculus runs medially along the entire length of the spinal cord and contains the fibers conveying from ascending fibers from the sacral, lumbar and lower six thoracic spinal nerves and the cuneate fasciculus runs laterally and only in the upper thoracic and cervical segments of the spinal cord and contains ascending fibers from the upper six thoracic and all the cervical nerves (9, 10).
The gracile fasciculus in the posterior or dorsal column of the spinal cord consists of fibers originated from S5 to T5 and organized in medio-lateral direction: Sacral fibers from approximately the S5 fifth sacral level (S); Lumbar fibers conveying input from lower extremity (L); Thoracic fibers conveying input from trunk approximately the fifth thoracic level (T).
The cuneate fasciculus in the posterior or dorsal column of the spinal cord consists of fibers originated from above T6-C1 and organized somatotopically in medio-lateral direction: fibers conveying from upper extremity; fibers conveying input from neck; fibers from approximately the second cervical level.
The medial lemniscus or bulbothalamic tract originates in the medulla oblongata and consists of the axons of the neurons of the gracile and cuneatus nuclei that go anteriorly, cross the midline and form the medial lemniscus running contralaterally from which they originate (Fig. 1B).
The medial lemniscus in the medulla oblongata, in relation to the spinal cord, undergoes an anticlockwise rotation so that the fibers have a dorso-ventral direction: the lumbar fibers are located more ventrally; the thoracic and upper arm fibers are in the intermediate zone; the cervical fibers are located more dorsally (Fig. 1C).
The medial lemniscus in the pons undergoes by a clockwise rotation, so that its fibers present a medio-lateral direction: the lumbar fibers convey input from lower extremity are located more laterally; the thoracic and upper arm fibers are in the intermediate zone; the cervical fibers are located more medially (Fig. 1D).
The medial lemniscus in the mesencephalon still rotates clockwise for 15°, so the medial lemniscus is located posteriorly to the substantia nigra and has oblique orientation: dorsal-anterior and latero-medial direction “LTA”. L: lumbar fibers conveying input from lower extremity are more dorsally and laterally; T: thoracic fibers conveying input from trunk are intermediate; A: arm fibers conveying from upper extremity are more ventrally or anteriorly and medially, located dorsally and laterally to red nucleus (Fig. 1E).
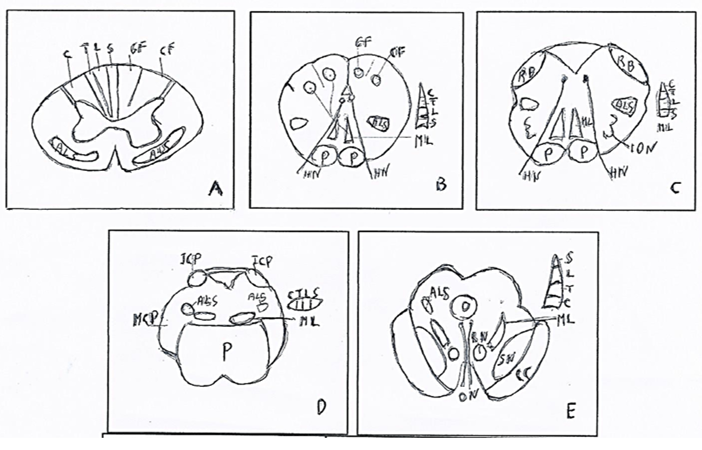
Figure 1: Medial lemniscus (ML): origin in spinal cord and course in braistem. A. Spinal cord. Somatotopy of lateral cuneate fasciculus (CF) with cervical fibers (C) and medial gracile fasciculus (GF)) with lateral-medial fibers: Trunk (T), Lumbar (L), Sacral (S) B. Caudal Medulla oblongata. Somatotopy of cuneate and gracile nuclei and origin of the medial lemniscus (ML) with somatotopy of the fibers: Cervical (C), Trunk (T), Lumbar (L), Sacral (S), in posteroanterior direction. C. Rostral Medulla oblongata. Somatotopy of the medial lemniscus (ML) with somatotopy of the fibers: Cervical (C), Trunk (T), Lumbar (L), Sacral (S), in posteroanterior direction. D. Pons. Somatotopy of the medial lemniscus (ML) with somatotopy of the fibers: Cervical (C), Trunk (T), Lumbar (L), Sacral (S), in mediolateral direction. E. Mesencephalon. Somatotopy of the medial lemniscus (ML) with somatotopy of the fibers: Cervical (C), Trunk (T), Lumbar (L), Sacral, in mediolateral and anteroposterior direction.
ALS: Anterolateral System. CC: Crus cerebri. CN: Cuneate nucleus. GN: Gracile nucleus. HN: Hypoglossal nerve. ICP: Inferior cerebellar peduncle. ION: Inferior olivary nucleus. MCP: Middle cerebellar peduncle. ML: Medial lemniscus. ON: Oculomotor nerve. P: Pons. p: Pyramid. RB: Restiform body. RN: Red nucleus. SN: Substantia nigra. From BURT (10) modified.
The somatotopy of the medial lemniscus in brainstem is showed in tab 1.
| Direction | Spinal cord | Medulla oblongata | Pons | Mesencephalon |
MEDIOLATERAL
| Lumbar T6 Trunk Arm Neck LTAN | Arm Trunk Lumbar ATL | ||
DORSOVENTRAL
| Arm Neck Trunk Lumbar A T L | |||
DORSOVENTRAL & LATEROMEDIAL
| Lumbar, Trunk, Arm L T A |
Table 1: Somatotopy of the medial lemniscus in brainstem.
Medial lemniscus contains fibers of an aberrant pyramidal tract and ends in the thalamus.
The thalamus is a diencephalic structure that is subdivided into groups of nuclei (Fig. 2).
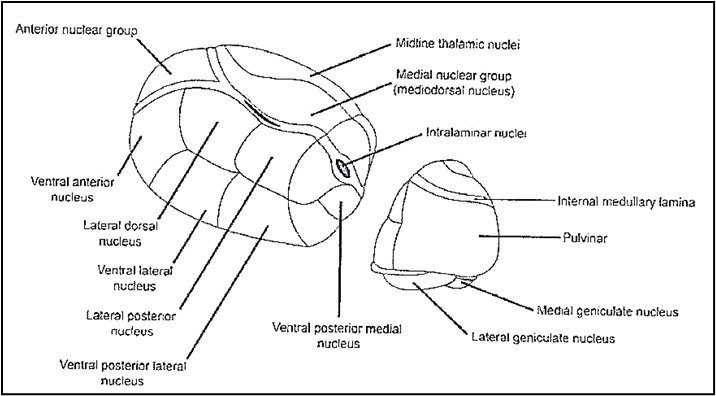
Figure 2: Groups of thalamic nuclei. The Ventral Posterior Lateral nucleus (VPL) (arrow) receives the sensory fibers.
1. Anterior group with the ventral anterior, anteromedial, anterodorsal nuclei that receive fibers from the mammillary nuclei, basal forebrain and brainstem and project fibers to the anterior limbic cortex of the cingulate gyrus and parahippocampal gyrus (which includes the medial pre- and parasubicular entorhinal cortex).
2. Medial group with the dorsomedial or mediodorsal nucleus subdivided in two partes:
2a. Magnocellular or anteromedial pars which receives olfactory fibers from piriform cortex, fibers from ventral pallidum, amygdaloid complex and projects the efferent fibers to anterior and medial prefrontal cortex.
2b. Parvocellular or posterolateral pars which has reciprocal connections with the prefrontal dorsolateral and dorsomedial cortex, anterior cingulate cortex, nonprimary motor area and posterior parietal cortex.
3. Lateral group subdivided in two subgroups: lateral and ventral.
3a. The lateral subgroup is subdivided in three partes: lateral-dorsal, lateral-posterior, pulvinar.
3b. The ventral subgroup is subdivided in three partes: ventral-anterior; ventral-lateral, ventral-posterior.
3b-p1. The ventral subgroup, pars ventral-anterior, ulteriorly, is distinguished in two parts: magnocellular pars which receives the fibers from the substantia nigra and sends fibers to frontal eye fields or area 8 Brodmann; main pars which receives fibers from the pallidus internus and sends efferent fibers to intralaminar nuclei and diffuse areas of the frontal lobe and anterior parietal cortex or area 6 Brodmann.
3b-p2. The ventral subgroup, pars ventral-lateral is distinguished in two parts: pars oralis which receives fibers from the ipsilateral pallidus internus and sends efferent fibers to the supplementary motor cortex and premotor lateral cortex; pars caudalis which receives afferent fibers from contralateral cerebellar nuclei, the spinothalamic tract and, also, the vestibular nuclei. The pars caudalis projects afferent fibers to primary motor cortex, in particular the its medial pars projects to area 4 in region of the head, while its lateral pars projects to the cortical area of the leg. The pars caudalis presents same somatotopy than the ventral-posterior nucleus. Both the two partes receive efferent fibers from cortical prefrontal areas.
3b-p3. The ventral subgroup, pars ventral-posterior receives the sensory tracts and is subdivided in the lateral pars (VPL) which receives the medial lemniscus or bulbothalamic tract and medial pars (VPM) which receives the trigeminothalamic tract.
The ventral posterior nucleus (VP) presents a large single, systematic representation of the body surface which occupies most or all of the ventral-posterior nucleus. The representation of the regions varied in extent according to magnification factor and position in the map, the magnification of various skin surfaces was variable so some skin surfaces especially the tips of the digits occupied relatively portions of the nucleus, while other skin surfaces such as the trunk active little tissue.
The ventro-posterolateral nucleus (10) (Figure. 3A) has the representation of the both trunk and extremities. Particularly on the lamina superior of the nucleus are represented, in medio-lateral direction, skull, ear, neck, shoulder, upper limb where its ventral part is located medially and its dorsal part laterally, thorax, dorsal trunk, lower limb where its ventral part is located medially and its dorsal part is located laterally, the glutei are medially.
The hand representation (Figure. 3B) is in ventral-anterior pars of the ventro-posterolateral nucleus with the digits 1-5 [1-5] in medio-lateral direction and dorsoventrally from the proximal to distal on each digit.
The representation of digit 1, typically, is less extensive than digit 2 and not cover the entire medial wall of the subnucleus. The representations of the digit tips occupy a large band of tissue that extends from the base of the nucleus to form its rostral wall. Thus, digit tips are found dorsorostrally as well as ventrally in VPL. Middle and proximal phalanges are represented more dorsocaudally in the nucleus. The representation of the palmar pad forms most of the dorsocaudal cap of subnucleus A, with ulnar pads laterally next to ulnar digits. The representations of the dorsum and wrist in subnucleus A form a junction dorsally with the part of subnucleus that is devoted to forearm. The dorsal hairy surfaces of the digits occupy little tissue and are found in part caudoventrally and in part rostrodorsally near the representations of the glabrous distal phalanges.
The “foot” representation in subnucleus is much like the representation of the hand with any differences that the disclike representations of the digits of the foot are rotated slightly so that the rostral portion of each disc is medial to the caudal portion (Figure. 3C).
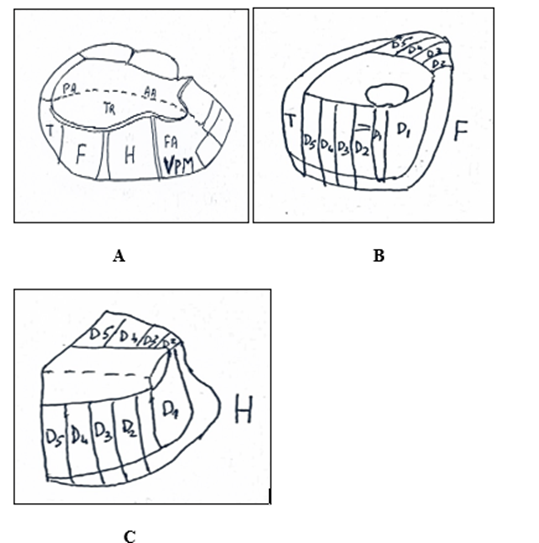
Figure 3. Thalamus. A. Somatotopy of the medial lemniscus and anterolateral system in ventral posterolateral nucleus (VPL) in which trunk fibers terminate and posteromedial nucleus (VPM) in which face fibers terminate. B. Somatotopy of foot in VPL. C. Somatotopy of hand in VPL.
H: Hand. F: Foot. T: Tail. Tr: Trunk. AA: Anterior arm. PA: Posterior arm. VPM (Ventral posteromedial). FA: Face. D: Digitus 1,2,3,4,5. From: Burt (10) modified.
The thalamic ventral posterolateral nucleus consists of the neurons which synapse with the fibers of the medial lemniscus and are organized somatotopically, as in the mesencephalon, in direction laterally to medially, L (Lumbar) T (Trunk) A (Arm) (Hainer). These neurons of nucleus project to cerebral somatosensory cortex by thalamocortical fibers (Figure. 4A).
The thalamic ventro-posteromedial nucleusVPM consists of the neurons which synapse with the fibers of the trigeminothalamic tract and has the representation of the face: the superior labrum is its lateral part; in the part inferior and medial of this lateral part are the teeth, more precisely the teeth of upper jaw are superior and those of the inferior jaw are inferior. In the medial part of the postero-medial nucleus in medial-lateral direction is represented the inferior labrum, the angle of the jaw and the mentum. These neurons of thalamic nucleus project to the cerebral cortex by thalamocortical fibers.
The Thalamus gives rise to thalamocortical fibers that run in the internal capsule (Figure. 4B), the lamina of white substantia that is located between the thalamus medially and striatum laterally and is subdivided in the anterior limb, genu, and posterior limb.
The sensitive thalamocortical fibers of both medial lemniscus and spinothalamic tracts run in posterior third of the posterior limb and arrive to primary somatosensory cortex. The orientation is inverted in direction laterally-medially LTA (Figure. 4A) and the fibers L project to posterior paracentral gyrus in the medial surface of the cerebral hemisphere, the fibers T project to postcentral gyrus medially and fibers A project to postcentral gyrus laterally.
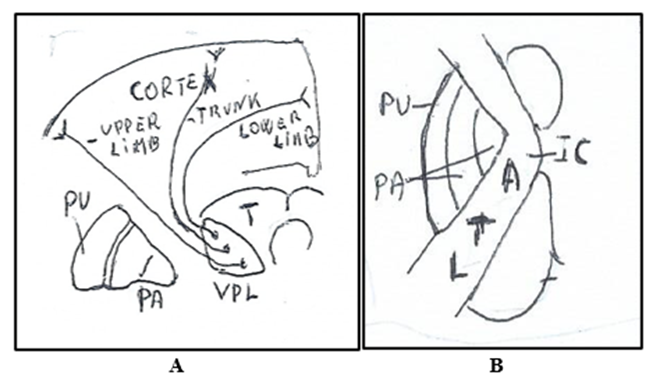
Figure. 4 A. Medial Lemniscus in Thalamus (T) in ventroposterolateral nucleus (VPL) with the thalamocortical fibers of the lower limb posteriorly, fibers of trunk in intermediate position and fibers of upper limb anteriorly. All fibers terminate in the somatosensory cortex. B. Medial lemniscus in Internal capsule (IC). The thalamocortical fibers with disposition in antero-posterior (ATL) and medial-lateral (ATL) direction. A: Upper extremity regions. T: Thoracic regions. L: Lower extremity regions. PU: Putamen. PA: Globus pallidus.
Medial lemniscus: Clinical aspects (10).
Lesion of the unilateral medial lemniscus in the medulla oblongata causes deficit or loss of deep sensation (pressure, joint and vibratory sensitivity), reduced discriminative sensitivity of the contralateral hemisome, if the lesion is rostral at the decussation of the lemnisci. The lesion in the pons is bilateral because the two medial lemnisci are very close to each other. Concomitant symptoms are: paralysis or peripheral paresis of the ipsilateral tongue.
If the lesion extends ventrally, it affects the pyramidal bundles and therefore there is hemiplagia on the side opposite to those of the hypoglossal lesion and therefore there is paresis of one or both upper limbs and much less of the lower limbs. Lesions of the midbrain can cause paresis due to concomitant oculomotor nerve.
Anterolateral System
The Anterolateral System (ALS) is a composite bundle constituted of the afferent fibers that originate in the spinal cord and end in: reticular formation by spinoreticular fibers, mesencephalon by spinotectal fibers ending to deep layers of the superior colliculus and by spino-periaqueductal fibers ending to the periaqueductal grey, hypothalamus by spinohypothalamic fibers, thalamus by spinothalamic fibers ending in the lateroventral posterior nuclei of the dorsal thalamus.
The ALS includes also the spino-olivary tract projecting to the accessory olivary nuclei.
The spinothalamic tracts (1) originate in the spinal cord and are constituted of fibers or neurites of the second-order neurons, present somatotopy and convey information about temperature and pain, touch non discriminative, baresthesia to the thalamus. Studies (11) on human cervical spinal cord evidenced that in the high cervical spinal cord, spinothalamic fibers mediating sharp pain for the arms are located ventromedial to fibers for the legs, and these fibers are spatially distinct from fibers that mediate heat pain.
We consider the somatotopy of the two anterior and lateral spinothalamic tracts which are two distinct fascicles, though recent physiological studies indicate that they are to consider a “single morphofunctional system”.
The anterior spinothalamic tract is located in the anterior funiculus of the spinal cord but proceeding rostrally in the neuromeres in the anterolateral position. At the lumbar level, this tract is located anterolaterally to the reticulospinal tract, posteroanterolaterally to the vestibulospinal tract and laterally to the anterior or direct corticospinal tract; at the cervical level, however, it is located medial to the anterior spinocerebellar tract, superior to the spino-olivar tract, inferior to the rubrospinal tract and lateral to the lateral reticulospinal tract.
The anterior spinothalamic tract in the spinal cord (9) is constituted of fibers somatotopically arranged in the two directions:
lateromedial direction, so fibers originating from the most caudal segments are situated laterally and fibers originating from more rostral segments are situated more medially.
Anteroposterior direction so the superficial (ventral) fibers are originated from the sacral segments, the deeper (dorsal) from cervical segments.
The anterior spinothalamic tract, in relation to its functionality, presents the somatotopy in medial-lateral direction: the pressure-sensitive fibers are medial; the non-discriminative or crude touch fibers are lateral.
The pathway of the anterior spinothalamic tract is similar to that of the lateral spinothalamic tract. Its fibers originate in the posterior horn of spinal cord from a second-degree neuron located lateral to the central canal that synapte with the central process of the first-degree neuron located in the spinal ganglion. The axons of the second-degree neurons through the anterior white commissure pass contralaterally, form the contralateral anterior spinothalamic tract and end in the ventral posterolateral nucleus of the thalamus (Fig. 5A).
Spinothalamic tracts are made up of the axons of neurons located in the spinal laminae that play different roles.
Spinal lamina I neurons receive and transmit afferents that affect pain and temperature, with small receptive fields, are of the high-threshold type or to a lesser extent wide dynamic range.
Spinal laminae IV-VII are mostly wide dynamic range, partly high threshold and small part low threshold. They carry cutaneous or non-painful pain stimuli.
Spinal laminae VII-VIII have a wide receptive field, are mostly high threshold, minor to wide dynamic range and small part low threshold.
The fibers of the bundle ascend; thus, the bundle enlarges through the entire spinal cordand brainstem terminating in the thalamic ventral posterolateral and the lateral central intralaminar nuclei. From here, third-degree neurons are distributed, after passing through the internal capsule, to the primary somatosensory cortex, respecting a somatotopism.
The Anterior spinothalamic tract in brainstem: medulla oblongata, pons mesencephalon.
Anterior spinothalamic tract in the medulla oblongata is located dorsolaterally to the inferior olivary nucleus, at level through the upper pons and midbrain, it becomes closely associated with the medial lemniscus. At medullary levels some fibers or collateral project into brainstem reticular formation, some terminate about cells of the lateral reticular nucleus of the medulla, a cerebellar relay nucleus. The anterior spinothalamic tract contains some fibers originating from neurons of the gracilis nucleus that send their axons into the medial lemniscus together with the gracilis fasciculus and the cuneate, then at the level of the medulla oblongata or bulb and pons, these fibers rejoin the anterior spinothalamic tract, detaching from the medial lemniscus and ascend with the former. The anterior spinothalamic tract in medulla oblongata maintains the same latero-medial direction order (SLTA) than the spinal cord, so fibers originating from the most caudal segments are situated laterally and fibers originating from more rostral segments are situated more medially. (Figure. 5B, C).
In the pons the ALS is located posteriorly and laterally to medial lemniscus and its somatotopic order of fibers maintains the lateromedial direction (LTA), as in medulla oblongata, so fibers originating from the most caudal segments are situated laterally and fibers originating from more rostral segments are situated more medially (Figure. 5D).
In the mesencephalon the ALS is located posteriorly and laterally to medial lemniscus and anteriorly to the spinotectal tract. The fibers of ALS are in anteroposterior direction: L: Lower extremity regions, T: Thoracic regions, A: Upper extremity regions tract (Figure. 5E).
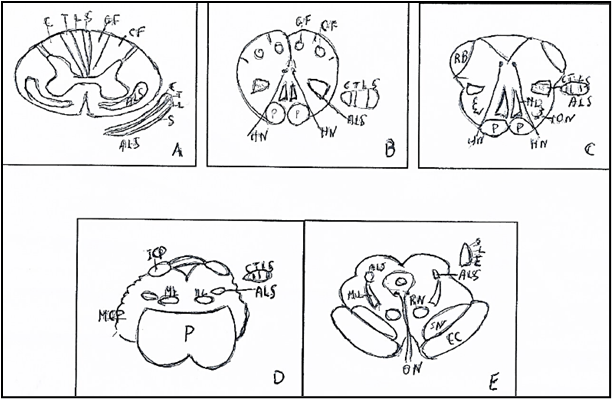
Figure 5: Somatotopy of Anterolateral system (ALS) in spinal cord and in braistem. A. Spinal cord. Somatotopy of Anterolateral System (ALS) with: Cervical fibers (C), Trunk (T), Lumbar fibers (L), Sacral fibers (S). B. Caudal Medulla oblongata. Somatotopy of Anterolateral System (ALS) with fibers, in latero-medial direction, of the regions: Sacral r. (S), Lower extremity r. (L), Thoracic r. (T), Upper extremity r. (A). C. Rostral medulla oblongata. Somatotopy of Anterolateral System (ALS) with fibers, in latero-medial direction, of the regions: Sacral r. (S), Lower extremity r. (L), Thoracic r. (T), Upper extremity r. (A), D. Pons. Somatotopy of Anterolateral System (ALS) with fibers, in in latero-medial direction of the regions: Sacral r. (S), Lower extremity r. (L), Thoracic r. (T), Upper extremity r. (A). E. Mesencephalon. Somatotopy of the Anterolateral System (ALS) with fibers, in antero-posterior direction, of the regions: Cervical r. (C) Trunk r. (T), Lumbar r. (L), Sacral r. (S).
ALS: Anterolateral System. CC: Crus cerebri. CF: Cuneate fasciculus. CN: Cuneate nucleus. GF: Gracile fasciculus. GN: Gracile nucleus. HN: Hypoglossal nerve. ICP: Inferior cerebellar peduncle. ION: Inferior olivary nucleus. MCP: Middle cerebellar peduncle. ML: Medial Lemniscus. ON: Oculomotor nerve. P: Pons. p: Pyramid. RB: Restiform body. RN: Red nucleus. SN: Substantia nigra. From BURT (10) modified.
In the thalamus the ALS terminates in the ventroposterolateral nucleus (VPL) that in mammals is somatotopically organized where the forelimb areas more medial than the hindlimbs (Figure. 6).
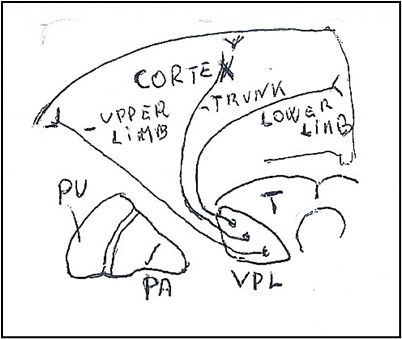
Figure 6: Spinothalamic tract in thalamic ventroposterolateral nucleus (VPL) with the thalamocortical fibers of the lower limb are posteriorly, fibers of trunk are intermediate and fibers of upper limb are anteriorly. All fibers terminate in the somatosensory cortex. PU: Putamen. PA: Globus pallidus.
Besides the VPL nucleus can be divided into subregions that receive different sensory modalities with varying receptive field sizes; for example, in the rat, the rostral ventroposterior lateral (VPL) nucleus receives mainly proprioceptive information with large cutaneous receptive fields on distal limb segments, the middle portion of the VPL is mostly cutaneous with small receptive fields, while the caudal VPL mainly receives cutaneous inputs with large receptive fields. Also, AA found the somatotopic locations for hand and leg of the spinothalamic pathway (STP) at the VPL nucleus and these somatotopies were arranged in the anteroposterior direction [12].
In the internal capsule (Figure. 7A) the thalamocortical fibers originating from the thalamic ventroposterolateral nucleus run in its posterior limb with fibers that are in order in direction antero-posterioly (ATL) and from medial-laterally (ATL): Upper extremity regions (A), T: Thoracic regions (T), Lower extremity regions (L). and terminate in the primary somatosensory cerebral cortex. The thalamocortical fibers running in internal capsule end in cerebral cortex.
In the cerebral cortex (Figure. 7B. the thalamocortical fibers end in the somatosensory areas which present a distorted representation, homunculus, of the human body with each part extended, not in relation to its bodily extension, but in relation to its sensitivity or motility. Thus, the areas that are finely controlled, such as the digits, have larger portions of the somatosensory cortex, whereas areas that are coarsely controlled, such as the trunk, have smaller portions.
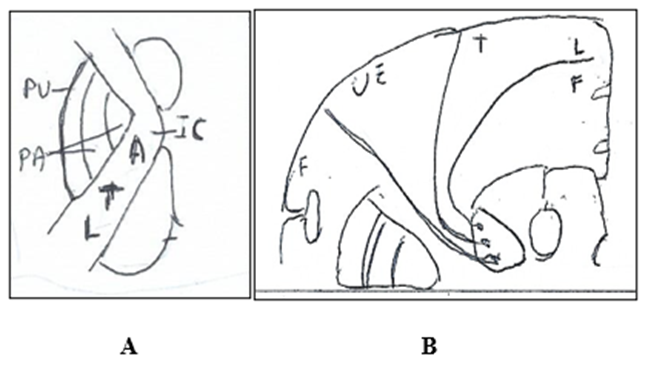
Figure 7: Somatotopy of the thalamocortical fibers. A. Internal Capsule (IC) The thalamocortical fibers are in antero-posterior (ATL) and in medial-lateral (ATL) direction. A: Upper extremity regions. T: Thoracic regions. L: Lower extremity regions. B. Cerebral Hemisphere. The thalamocortical fibers terminate in postcentral parietal gyrus distributing from the lateral surface to the medial surface in sequence: face, upper extremity, trunk, inferior extremity. F: Face. UE: Upper extremity. T: Trunk. L: Leg. F: Foot.
The cerebral cortex (10) presents three somatosensory areas: primary S-I; secondary S-II, retroinsular area. (Figure. 8).
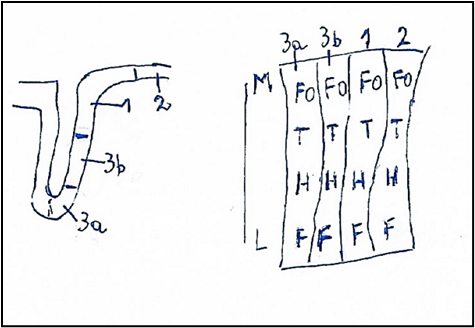
Figure. 8: Cerebral cortex. Somatotopy of the cerebral somatosensory area: Central sulcus or Sulcus of Rolando and Postcentral Gyrus with the somatosensory areas: 3a, 3b, 1, 2. The Somatosensory Areas are showed in extension and in mediolateral direction M-L. M: Medial. L: Lateral, FO: Foot, T: Trunk, H: Hand, F: Facies. From BURT (10) modified.
The primary somatosensory area [13] is located in the postcentral gyrus of the parietal lobe and includes four distinct representations of the body: 3a, in the depth of the central scissure, 3b more extended for most part of the posterior wall of the central scissure, 1 that is located in crest of the central scissure, 2 that is the more caudal of the area S-I.
Each somatotopic map extends in medial-lateral direction and is organized according to the scheme similar to the classic homunculus of Penfield and Rasmussen: the foot and lower limb are located medially and are followed by the trunk, upper limb, hand and face. as you move laterally and downwards on the surface of the postcentral gyrus in the anterior parietal cortex.
The S-I, area 3a is situated in the depth of the central scissure or sulcus of Rolando, receives fibers originating from VPS and conveying information from the neuromuscular spindles and deep of frontal cortex. The area 3a projects reciprocally with area 2 and S-II, has connections with motor and premotor areas of the adjacent frontal gyri.
The S-I, area 3b is called the S-I area proper; it is inseparable for tactile discrimination and appreciation of forms receiving information from cutaneous mechanoreceptors and is reciprocally connected with areas 1,2 and S-II.
The S-I, area 1 receives information from both cutaneous mechanoreceptor’s trough neurons of the VP nucleus and is reciprocally connected with areas S-I 2-3b and S-II.
The S-I,area 2 receives information from both cutaneous and deep receptors, neuromuscular spindle by neurons of the VPS nucleus and projections from the cortical areas 3b and 1.
Spinothalamic lateral tract
The lateral spinothalamic tract [1, 13] is located in the lateral funiculus of the spinal cord, medially to the anterior spinocerebellar bundle, inferiorly to the lateral corticospinal bundle. The tract originates from secondary neurons located of the spinal posterior cord at the base in laminae I, IV-VIIII, predominantly in laminae VI or VII. These secondary neurons synapse with the central process of the primary or protoneurons located in the spinal ganglion and send axons in the same neuromere or ascend some neuromeres and decussate across the anterior white commissure and then ascending from neuromere to neuromere accumulate to form the contralateral lateral spinothalamic tract.
The lateral spinothalamic tract or paleospinothalamic tract conveys pain and thermal sense; it originates from neurons of spinal laminae VI, VII, VIII of Rexed, its fibers cross to the opposite side within one segment and have a somatotopic lamination the most lateral and posterior fibers represent the lowest portion (sacral) of the body, whereas the more medial and anterior fibers are related to the upper extremity and neck (cervical).
In C8, also, there is also lamination of the sensory modalities; so, the fibers concerned with the heat pain (thermal sense) are located posteriorly, while the fibers associated with the sharp pain are located more anteriorly and in both the fibers from hand are medially while the fibers from foot are laterally.
In the brainstem the two anterior and lateral spinothalamic tracts are separate:
The spinotalamic anterior tract goes together the lemniscus medial or bulbo-thalamic tract, (neospinotalamic tract) without interruption to thalamic levels.
The spinothalamic lateral tract continues into the spinal lemniscus comprising also the spinothalamic and spinotectal tracts.
in both medulla oblongata and the pons, the somatotopy is same and some fibers of the bundle, at the level of the medulla and pons, synapte in the reticular formation.
in the mesencefalon the fibers the lemniscus spinal conveying the thermal and pain sense from lower arm extend dorsally, while the fibers from the trunk and upper arm are ventrally.
in the thalamus the anterior and lateral spinothalamic tracts project to the ventral-posterolateral thalamic nucleus and to the central intralaminar nucleus; these thalamic nuclei give rise to the thalamocortical fibers that terminate by distributing in the primary somatosensory cortex respecting the somatotopic organization already described for the dorsal columns.
The anterolateral system, also, includes the spinoreticular and mesencephalic fibers.
The ascending spinoreticular fibers originate from neurons located in all neuromeres of the spinal cord in laminae V, VII and VIII and constitute a bundle placed in the context of the spinothalamic tract with which it runs. The bundle ascends into the bulb, where it receives axons from the lateral reticular nucleus and the nucleus of the spinal tract of the trigeminal nerve, and at the same time some synapte fibers in the reticular formation. It goes further up into the pons, where some endings still synapse in the reticular formation and receives ascending fibers from the same formation; the same thing happens in the midbrain where the bundle receives fibers of neurons of the mesencephalic tegmentum. Thus, most of the fibers of the ascending spinoreticular bundle are distributed to the hypothalamus and to the intralaminar nuclei of the thalamus. It does not seem to have somatotopic organization and seems to be involved in the transmission of pain stimuli, but also in skin and deep sensitivity.
The spinomesencephalic fibers originate in neurons located in laminae I and IV-VIII (V is the one that gives rise to most of the fibers) in the spinal cord and form bundles that project to the midbrain contralaterally. Among these bundles, the most important are the spinotectal bundle that runs together with the lateral spinothalamic bundle, the fibers that terminate in the periaqueductal gray matter of the midbrain, fibers that lead to the parabrachial nucleus, the pretectal nuclei and the Darkschewitsch nucleus located anterior to the oculomotor nucleus. Most neurons carry pain information.
Clinical aspects of the spinothalamic tracts
The spinothalamic tract is an anterolateral pathway located on the same side of the body, so an injury on this side of the body will cause a deficit of everything that is controlled below the point of injury with loss of pain and temperature sensations on a contralateral side of the body about two levels below the injury. Such lesions occur in bone marrow or Brown-Sequard syndrome, syringomyelia in which the anterior white commissure is obliterated due to cavitation in the central spinal cord, and acute cervical spinal cord syndrome.
The spinothalamic tract can also be compromised due to vascular problems: an example is the anterior spinal artery infarction syndrome in which there is pain and loss of temperature bilaterally below the level of the lesion, while the vibratory and proprioceptive senses are preserved due to the sparing of the posterior spine. Injury of the anterolateral fascicle (10) causes loss of pain and thermal sensitivity, while tactile sensitivity is preserved, but really this sensitivity is also altered since the spinothalamic tracts contain also tactile fibers.
Pyramidal System
The knowledge of the somatotopy of the pyramidal system, pathway of the voluntary movement, is important in clinical implications for neurosurgery and neurology.
The pyramidal system is the major or main or preeminent voluntary motor pathway that controls voluntary movements in humans and encloses the corticospinal and corticobulbar tracts which originate in the cerebral motor primary area and terminate in the spinal cord and brainstem respectively.
The somatotopic location of the corticospinal tract is studied along its course from the cerebral cortex to the spinal cord.
The corticospinal tract (CST) is constituted of the efferent fibers originated from the internal pyramidal cell layer or layer V of the primary motor cortex that is the precentral gyrus in the posterior part of the frontal lobe of the cerebrum, but some fibers of both corticobulbar and corticospinal tracts come from the pre-motor area and supplementary areas. These efferent fibers form the corona radiata and pass in the internal capsule.
The CST in the corona radiata (CR) is located posteriorly and, also, the CST for the hand descended through about one quarter (medio-lateral direction) and two-thirds (antero-posterior direction) at the CR (14). Studies (15) on the CR of a normal human brain by diffusion tensor tractography suggest that the anatomical location and somatotopic organization for the upper extremity are found anterolaterally to the lower extremity and the distances between the upper and lower extremities become decreased as the CST descends from the upper to the lower CR level.
The internal capsule (IC) (Fig. 9) consists of descending and ascending fibers tracts. It is generally thought that the two main descending fibers tracts, the corticobulbar tract and the corticospinal tract (CST), descend separately through the genu (16) and through the anterior third of the posterior limb of the internal capsule (PLIC) [17; 18].
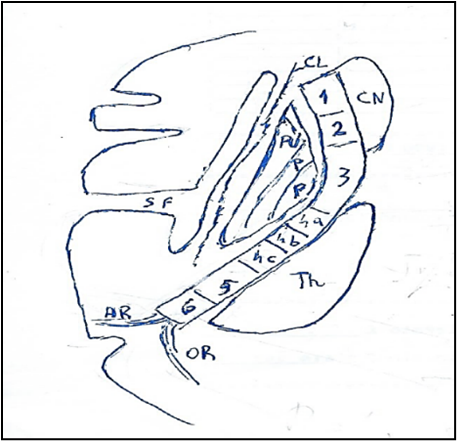
Figure 9: Corticospinal tract in Internal capsule.
The internal capsule in anteroposterior direction is subdivided: anterior limb, genu, posterior limb. Anterior limb presents: 1. Anterior thalamic radiation; 2. Frontopontine fibers. Genu presents: 3 Corticobulbar tracts. Posterior limb presents: 4a Corticospinal tract to upper limb, 4b Corticospinal tract to trunk, 4c Corticospinal tract to lower. 5: Thalamoparietal fibers. 6 Temporopontine fibers. AR: Auditory Radiation. CL: Claustrum. CN: Caudate Nucleus. OR: Optic Radiation. P: Pallidum. PU: Putamen. SF: Sylvian fissure. Th: Thalamus.
Studies [19] by diffusion-tensor MR tractography found that the CSTs lie in a compact area in one part of the PLIC; if the PLIC is divided into four equal quarters from anterior to posterior, the CST was shown to be in the third quarter. CSTs, 17 of 20 were organized somatotopically, with hand fibers anterolateral to foot fibers, not anteromedial as is currently believed. In three of 20, hand and foot fibers were intermixed. The AA confirm some recent results that the CST is located more posteriorly, but not in the anterior third as it was thought classically.
Studies [20] in the patients with an infarction in internal capsule showed that the hand fibers are located laterally to foot fibers in the short axis of the posterior limb of the internal capsule (PLIC) and the face fibers are located in the premedial part of the PLIC. The motor subtracts in cat [21] are aligned along the long axis of the PLIC in anteroposterior orientation. The tongue pathways were located anteromedial to the face; the face, anteromedial to the hand; and the hand variably, anterior to the foot.
In Cerebral Peduncle (CP) the corticospinal tract (CST) (Fig. 10) is located in the middle to lateral portion. between the corticobulbar tract medially and the cortico-temporo-pontine or tract of Turk laterally. Studies in human brain [22, 23] using DTT (diffusion tensor imaging) with FSL tools (Functional MR Imaging of the Brain Software Library) observed that the CST showed transverse orientation in the middle to lateral portion of the CP, and that hand fibers are located medially respect to foot fibers that are located laterally. Therefore, AA concluded that in CP the somatotopic arrangement for the hand and leg shows medio-lateral orientation.
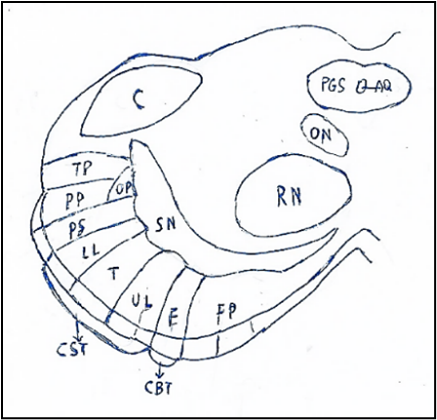
Figure. 10. Crus of cerebral peduncle. Somatotopy of the corticospinal (CST) and corticobulbar (CBT) tracts. The cerebral peduncle in medial-lateral direction: Frontopontine fibers, Facies, Upper limb, Trunk, Lower limb, Parietospinal fibers, Parietopontine fibers, Occipitopontine fibers. Temporopontine fibers,
AQ: Aqueduct of Sylvius. C: Colliculus. F: Facies. FP: Frontopontine fibers. LL: Lower Limb. ON: Oculomotor Nucleus. OP: Occipitopontine fibers. PGS: Periaquaeductal gray substance. PP: Parietopontine fibers. PS: Parietospinal fibers. SN: Substantia nigra. RN: Red Nucleus. TP: Temporopontine fibers. T: Trunk. UL: Upper Limb.
In the Pons the corticospinal tract (CST) [24] is located in the center of the pontine basis, which is surrounded by transpontine fibers. AA discovered that fibers for arm motor function were located antero-medially, while those for leg motor function were located more postero-laterally at the pons in 17 stroke patients with brainstem lesions. Levi and Schwalb [25] using DTT in normal human brains observed that the location of regard the somatotopies for the hand and leg and they found that “hand somatotopy” descended through the ventro-medial portion of the pontine basis and that “leg somatotopy” was located dorso-laterally to the “hand somatotopy” of the CST.
Recent studies [26] conclude that a topographical arm/ leg distribution of the CST is lost as the CST fibers descend through the pons. The combination of tract-tracing studies and in vivo (MRI) tract localization studies provides strong evidence that the CST is somatotopically organized within the cerebral cortex, as well as the internal capsule within the cerebral peduncle CP to some extent; however, there is no such organization of human CST fibers caudal to the pons or at the level of the medullary pyramid and spinal cord.
In the Medullary Pyramid (MP) the corticospinal tract (CST) from pons passes in the pyramids of medulla oblongata, it exits the pyramids in consecutive packages and approximately some fibers (90%) cross over or decussate the medulla oblongata’s anterior median fissure as pyramidal decussation or motor decussation and proceed down the spinal cord on the contralateral area at the pyramids most caudal end.
Fibers decussed (90%) migrate in spinal cord down the lateral corticospinal tract, whereas fibers not decussate (10%), remain ipsilateral and migrate down the anterior corticospinal tract. The decussated fibers of the lateral corticospinal tract induce motion on the part of the body that is contralateral to the hemisphere from which they arose; the not-decussed fibers (10%) of the anterior corticospinal tract keep going down into the ipsilateral spinal cord and the majority of the axons decussate in the spinal cord right before synapsing with lower motor nerve cells.
Investigations [27] on the somatotopic arrangement of the CST at the MP in 30 human normal brains by normalized DTT evidenced that the hand somatotopy of the CST was located in the medial portion of the MP; in contrast, the leg somatotopy occupied the lateral portion of the medullary pyramid.
In spinal cord, the literature data report the historical concepts of the CST with a laminar and somatotopic organization: arm medially, trunk intermediate, leg laterally (Figure. 11A). The current literature data by tract-tracing and in vivo MRI studies, selective ablations CTS, evolutionary assessment of the tract in mammals and neuropathological examinations of patients after incomplete cervical spinal cord injury involving the CST and prominent arm and hand dysfunction accumulate evidence that there is no somatotopic organization of the corticospinal tract within the spinal cord in humans (Figure. 11B) [26]. This concept was supported by studies [25] on macaque monkey demonstrating the presence of many intermingling of fibers originating from primary motor cortex arm/hand, shoulder and leg areas and also no significant difference in their distribution across different subsectors of the pyramidal tract or lateral corticospinal tract.
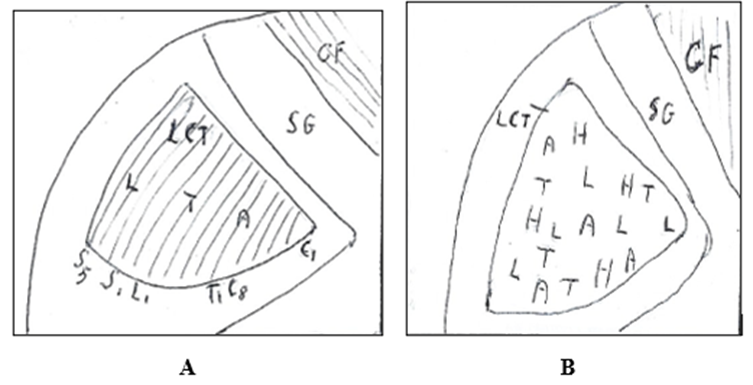
Figure 11. Spinal cord: Lateral corticospinal tract (LCT). A. Lateral corticospinal tract composed of laminar fibers that innerate in medial-lateral direction: arms, thorax, leg. From Gray’s modified. B. Lateral corticospinal tract composed of nonsegregated dispersed fibers that innervate the muscles of hand (H), arm (A), thorax (T) and leg (L). CF: Fasciculus cuneatus. SG: Substantia grisea. C1, C8, T1, L1, S1, S5: Spinal Segments. From: Levi and Schwab [25] modified.
Furthermore, AA found that fibers from the primary somatosensory cortex arm area completely overlap those from the leg area and both these outputs overlap those from posterior parietal sensorimotor areas, was demonstrated an intermingling with corticospinal outputs derived from premotor and supplementary motor arm areas and, also, was evidenced that no somatotopy for corticospinal projections from rostral and caudal cingulate motor areas and from somatosensory areas of the parietal cortex.
Clinical studies [26] show that in some spinal injury is greater deficit in upper that lower limb movement and this condition can be due to the complex organization of corticospinal tract and to a greater relative influence of the corticospinal tract on upper versus lower limb movements, the former best characterized by skilled hand and digit movements.
The corticospinal tract (CST) predominantly presents contralateral projections, though anatomical studies mapping CST connectivity in mammals provided evidence of ipsilateral CST (iCST) projections that descend in the lateral or ventral funiculi of the spinal cord of cats and monkeys or that are components of re-crossed contralateral fibers [28] (Figure. 12 o 19).
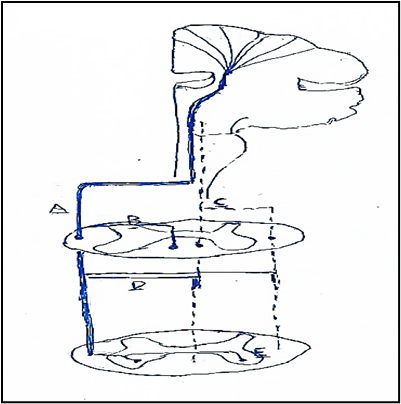
Figure. 12. Corticospinal (CST) projections into spinal interneurons. A. Most of CST fibers (full line) cross at the caudal medulla and descend through dorsolateral column. B. A smaller component of crossed CST fibers (full line) descends in the ventral column. C. Ipsilateral CST uncrossed fibers (dotted line) at the level of caudal medulla and descend through ipsilateral anterior and dorsolateral columns. D. Some crossed CST (full line) projections may re-cross the midline and descend ipsilaterally different spinal levels. E. Ipsilateral axonal innervation from crossed (full line) CST fibers through axonal collaterals that re-cross the midline and innervate the opposite spinal hemichord. From: Alawieh modified.
These iCST projections are sometimes referred to as “latent” projections since they not contribute to motor function in the presence of intact contralateral projections, but probably their role in cortical motor control may arise after a lesion to the crossed CST projections. In fact, unilateral lesions of the CST are associated with significant motor recovery which may suggest the existence or emergence of iCST projections in mammalian models and also in human patients. A potential role of iCST in recovery from injury would provide a rational for targeting these fibers to modulate the recovery process [29].
Besides, an aberrant pyramidal tract was observed; it originates from the primary motor cortex and the supplementary motor area, descends through the corona radiata, passes through the posterior limb of the internal capsule and in the medial lemniscus pathway from the midbrain to the pons, thus enters into the pyramidal tract area at the pontomedullary junction. Yeo and Jang [30] reported that a patient 21-year-old man with right hemiparesis due to a traumatic intracerebral hemorrhage in the left corona radiata had recovered the motor functions of the right extremities by this aberrant pyramidal tract.
Corticobulbar Tract
Corticobulbar tract supplies upper motor neuron innervation to the cranial nerves supplying head and face.
The corticobulbar tract starts in the lateral part of the precentral gyrus; from here, the corticobulbar fibers pass from lateral pars to medial pars of the corona radiata, pass in the genu of the internal capsule, medial part of the cerebral, basal part of the pons, and then to the medullary pyramid. The corticobulbar fibers peduncle synapse with motoneurons of the nuclei of the cranial nerves III, IV, V, VI, VII IX, X, XI, XII.
In the internal capsule that consists of descending and ascending fibers tracts, the descends through the genu separately from the corticospinal tract which descends through the anterior third of the posterior limb. The face fibers are located in the anteromedial part of the posterior limb of the internal capsule.
In the mesencephalon the corticobulbar tract passes in the cerebral peduncle and is located the fronto-pontine fibers medially and the corticospinal tract laterally.
In the pons the corticobulbar tract innervates the motor facial nucleus, Terao and coll. [31] hypothesized that the corticobulbar tract descends at ventromedial lower pons near the corticospinal tract, mainly at level of the upper medulla, where the fibers then cross and ascend in the dorsolateral medulla to synapse in the contralateral facial nucleus. Kanbayashi and Sonoo [32] found that the corticobulbar tract descends with the corticospinal tract at least to the middle portion of the medulla, and then ascends to the facial nucleus through the medial and ventral areas of the dorsolateral medulla after decussation.
In the medulla oblongata the corticobulbar tract innervates the motor nuclei of the glossopharyngeal and vagal nerves.
The corticobulbar tract provides input to the nuclei innervating muscles of the face.
The corticobulbar tract carries upper motor neuron input to motor nuclei of trigeminal, facial, glossopharyngeal, vagus, accessory, and hypoglossal nerves. The trigeminal motor nucleus supplies the muscles of mastication, The facial nerve supplies the muscles of facial expression. The glossopharyngeal and vagus nerves innervate the muscles of the pharynx, and larynx, The accessory nerve innervates the sternocleidomastoid, and trapezius muscles. The hypoglossal nerve innervates the contralateral tongue muscles (both intrinsic and extrinsic), except for the palatoglossus muscle.
The corticobulbar tract indirectly affects muscles of eyes supplied by third, fourth, and sixth cranial nerve through the medial longitudinal fasciculus and the paramedian part of the pontine formation.
The corticobulbar tract contains also fibers that end in the sensory nuclei of the brainstem including gracile nucleus, cuneate nucleus, solitary nucleus, and all trigeminal nuclei [32].
The corticobulbar tract innervates cranial motor nuclei bilaterally with the exception of the lower facial nuclei (which innervates facial muscles below the eyes) and the genioglossus muscle, which are innervated only unilaterally by the contralateral cortex. Among those nuclei that are bilaterally innervated a slightly stronger connection contralaterally than ipsilaterally is observed.
Jang and Kwak [34] report that an aberrant pyramidal tract (APT) was found in 17,9% and it descends through the medial lemniscus at the midbrain and pons level and then rejoined the pyramidal tract (PT) at the upper medulla. It is suggested that the APT could contribute to motor recovery following brain injury.
Also, Kwon and coll. [35] report that several characteristics of the APT described in comparison with the pyramidal tract (PT) in 93 normal subjects: the PTs always originated from the primary sensori-motor cortex, SM1: anterior boundary: precentral sulcus; posterior boundary: postcentral sulcus; medial boundary: midline; lateral boundary: lateral sulcus) while 26.5% of the APTs originated from the primary somatosensory cortex (anterior boundary: central sulcus; posterior boundary: postcentral sulcus; medial boundary; midline; lateral boundary: lateral sulcus) without a primary motor cortex (anterior boundary: precentral sulcus; posterior boundary: central sulcus; medial boundary: midline; lateral boundary: lateral sulcus) origin, APT descends along the known pathway of the PT to the posterior limb of the internal capsule, then descend through the medial lemniscus from the midbrain to the pons. The APT was separated from the PT at the upper midbrain level, the APT rejoined with the PT at the upper medulla level. These Authors concluded that the APT can be described as having less origin from the primary motor cortex, less directionality, and fewer neural fibers than the PT.
Clinical aspects
Lesions of pyramidal system
The injury on the pyramidal system can cause the pyramidal syndrome [36] with the damage to the neural pathways that control voluntary muscle movements and with loss of ability to perform coordinated movements, this condition can only occur in bulbar pyramids in which the pyramidal bundle has an isolated course. For pyramidal syndrome the "Babinski sign" may be significant by stimulating the sole of the foot with a pointed object that causes plantar flexion of the big toe and often also plantar flexion and adduction of the other toes, so if after stimulation there is dorsiflexion of the big toe or inverted plantar reflex is indicative of a pathological state.
Damage to the corticospinal tract will present similarly to upper motor lesion syndrome with symptoms such as spasticity, clonus, hyperreflexia, and Babinski sign. Primary symptoms of pyramidal tract injury is muscle weakness or paralysis (80%) on one side of the body that can manifest as difficulty in moving the arms, legs, or face on one side, and may also lead to a loss of coordination and balance. A common symptom is spasticity (65%) which is characterized by muscle stiffness and involuntary muscle contractions and consequently muscle cramps, pain, and difficulty in performing everyday tasks. Also, pyramidal tract injury can lead to sensory disturbances, such as numbness, tingling, or heightened sensitivity in the affected areas and still a cognitive and emotional symptom (40%) including difficulties with concentration, memory, and emotional regulation.
| Lesion of pyramidal system in: | Deficit in musculature of: |
| Gyrus prefrontal (Internal face). | Contralateral lower limb. |
| Gyrus prefrontal (External face) | Contralateral upper limb. |
| Internal capsule | Contralateral body including the head |
| Cerebral peduncle | Contralateral body and contralateral half head |
| Pons | Contralateral body and ipsilateral half head |
| Medulla oblongata: cranial to the decussation of the pyramids | Contralateral side |
| Medulla oblongata caudal to the decussation of the pyramids | Ipsilateral side. |
| Spinal cord: bilateral caudal decussation pyramids | Bilateral deficit of the body excluded head and both lower limbs |
| Spinal cord unilateral (right/left) caudal decussation pyramids | Ipsilateral deficits of the lower limb. |
Table 2: .
Injures to the corticospinal tract cause different damages in relation to the level at which the injury occurs (Tab. 2).
CST lesions (10) at the level of the brainstem between the red nucleus in the mesencephalon and the vestibular nuclei in the medulla oblongata there is decerebration rigidity as an exaggeration of the muscle tone and postural responses to sensory stimuli due to the absence of the superior controls. Noxious stimuli applied to the trunk and head cause extension of the axial muscles and extremities. Stimuli applied to the distal parts of the limbs cause exaggerated retraction of the flexors as much the cross-extensor reflexes. If the unilateral, the effects are ipsilateral; if the lesion is low, involving the lateral vestibular nuclei, the exaggerated hypertonia is eliminated as the vestibulospinal tract exerts an excitatory influence on the lower motor neurons, especially those innervating the extensor and antigravity muscles.
It is important to note that the severity and combination of symptoms can vary widely among individuals with pyramidal tract injury, depending on the location and extent of the damage. Some individuals may experience mild symptoms that only minimally impact their daily lives, while others may face more significant challenges in mobility, communication, and overall functioning. Lesion of the corticobulbar tract involved the lesions of the nuclei of cranial nerves.
Lesions of the oculomotor nuclei III, IV, VI [37] that control the ocular movements. The oculomotor (III) and trochlear (IV) nuclei control the vertical eye movements: a lesion unilaterally affecting the third-nerve nucleus results in an ipsilateral third-nerve paralysis and a contralateral upgaze paralysis because of the decussation of the superior rectus motoneurons, at the level of the third-nerve nuclei.
Nuclear lesions of third nerve [38] may cause isolated weakness of one of the muscles innervated by the oculomotor nerve, except the superior rectus muscle by its crossed innervation, the levator palpebrae superioris by a single caudal subnucleus innervating the levator muscles bilaterally, the pupillary constrictors (visceral nuclei are spread throughout the oculomotor nucleus), and the medial rectus muscle (three separate subnuclei). Thus, isolated unilateral palsies of the inferior rectus muscle have been associated with lesions of the inferior rectus subnucleus.
Trochlear nucleus
Lesion of the trochlear nerve (IV) [39] leads to ocular skew deviation and consequent diplopia. Subsequently, the brain ‘learns’ to compensate for the skew deviation by contralateral head tilt. The lesion is evidenced by the Bielschowsky test [40].
Abducens nucleus
Lesion of the abducens nucleus (VI) [41] produce paresis with an isolated ipsilateral abduction deficit and, however, because of the intranuclear connections, produce a conjugate gaze palsy toward the side of the lesion rather than just an ipsilateral abduction weakness that would occur from isolated damage to the abducens nerve. Lesions of the abducens nuclei bilaterally eliminate the horizontal conjugate gaze. Lesions of the abducens nucleus (VI) result in complete ipsilateral eye movement paralysis, and lesions damaging the Medial Longitudinal Fasciculus MLF result in internuclear ophthalmoplegia, whereas an association of these two lesions leads to the "one-and-a-half" syndrome.
Trigeminal motor nucleus
Lesions of the trigeminal motor nucleus V that is situated in the upper pons, inferior to the lateral part of the floor of the fourth ventricle. and contains motoneurons innervating the muscles originated by the first branchial arch: muscles of mastication (temporalis, masseter and pterygoids), muscles tensor tympani, tensor veli palatini, mylohyoid, and anterior belly of the digastric. The lesions of the trigeminal motor nucleus that forms the efferent pathway of the jaw-jerk reflex, thus a lesion involving the trigeminal motor nucleus would cause ipsilateral hemiparesis since the axons involved in this reflex do not decussate.
Facial nucleus
The upper motor neuron of the facial nerve VII [42] is located in the primary motor cortex of the frontallobe and its axons descend ipsilaterally as the corticobulbar tract via the genu of the internal capsule and reach the facial nucleus situated in the caudal portion of the ventrolateral pontine tegmentum, The neurons of facial nucleus originate axons that take an unusual course, traveling dorsally and looping around the abducens nucleus, where they form the facial colliculus at the base of the fourth ventricle, they travel along the ventral pons, medially to the spinal trigeminal nucleus, and emerge from the bulbo-pontine sulcus. It was demonstrated that the facial motor nucleus has a dorsal and ventral region: the neurons in the dorsal region innervate the muscles of the upper face and the neurons in the ventral region innervate the muscles of the lower face.
The motor root carries somatic motor fibers to facial expression muscles, stapedius, digastric posterior belly, and stylohyoid.
Facial paralysis (10) is weakening (paresis) or total paralysis of some or all of the muscles of mimic expression. Unilateral lesion is ipsilateral flaccid paralysis with disappearance of wrinkles and normal folds of the face giving a smooth and expressionless appearance, inability to close one eye, drooping of the corner of the mouth with loss of burrs.
Supranuclear palsy of the facial area spares the upper muscles (above the level of the eyelid rim) of mimic expression since the fibers of the corticobulbar tract that innervate the upper muscles are direct and crossed, while the fibers that innervate the lower muscles are only crossed, then the lesions of the corticobulbar tract produce contralateral paralysis of the lower muscles of mimic expression and spare the upper muscles: then patients can close both eyes and wrinkle both eyebrows and no muscle atrophy is observed.
Nuclear or Infranuclear Lesion
Lesions that involve the facial motor nucleus or the infranuclear portion of the facial nerve result in complete paralysis of all the facial muscles on the ipsilateral side. The patient presents with mouth droop, flattening of the nasolabial fold, inability to close eye, and smoothing of the brow on the damaged side.
Nucleus ambiguous
The nucleus ambiguus [43] has a rostral portion located posterior to the inferior olivary nucleus and contains second order motor cell nuclei that give rise to motor fibers of the glossopharyngeal nerve innervating the stylopharyngeus muscle. The nucleus ambiguus has a caudal part that give rise to fibers motor innervating the muscles of the pharynx and larynx. In addition, the nucleus ambiguus gives efferent fibers to the cranial part of accessory nerve (XI).
The nucleus ambiguus is involved in the motor functions of swallowing and speech. It gives rise to the efferent motor fibers of the vagus nerve (X) that innervate the muscles of the soft palate, larynx, pharynx, as well as the stylopharyngeus muscles and pharyngeal constrictor muscles of the glossopharyngeal nerve (IX). The muscles controlled by the nucleus ambiguus initiate the mechanism of swallowing and phonation.
The unilateral lesion of vagus nerve causes the permanent hoarse voice because of paralysis of the laryngeal intrinsic muscles, dysphagia for sagging of the soft palate ipsilaterally and deviation of the uvula moving away from the lesion since the activity of the contralateral muscles is intact. The lesion of the recurrent laryngeal nerve causes the hoarse voice, even if there is no involvement of the pharynx.
Unilateral lesions of the nucleus ambiguus may lead to dysphagia and hoarseness, and these characteristics are classic in lateral medullary syndrome (Wallenberg syndrome).
Bilateral lesions of the nucleus ambiguus completely paralyze the larynx, and the inability to move vocal cords during inspiration can cause death. Bilateral lesions are rare and most often seen in severe amyotrophic lateral sclerosis (ALS).
Accessory nucleus XI
The accessory nucleus (XI) innervates the sternocleidomastoideus and trapezius muscles.
Lesions of the upper motoneurons (10) induce paresis of the contralateral trapezius and sternocleidomastoid muscles because the descending corticospinal fibers cross in the decussation of the pyramids at the lower portion of the medulla oblongata before reaching the spinal nucleus of the accessory nerve. No atrophy of the muscles because the lower motor neurons of the spinal nucleus of the accessory nerve are intact.
Lesions of the inferior motoneuron induce paralysis or flaccid paresis and successive atrophy of the innervated muscles. Unilateral lesion induces asthenia of the sternocleidomastoid muscle. Lesion of entire accessory nerve or of its ramus to trapezius muscle induces paresis of the trapezius muscle, causes lowering of the shoulder and inability to lift the shoulder.
Hypoglossal nucleus XII
Supranuclear lesions (10) to the hypoglossal nucleus (XII) occur at the cerebral cortex, the corticobulbar tract of the internal capsule, cerebral peduncle or the pons. Supranuclear lesions cause the tongue to protrude away from the nerve because of predominant neural crossing for upper motor neurons, but these lesions do not tend to cause atrophy but can lead to an uncoordinated tongue with slow but spastic tongue movements. Lesions of the upper motoneuron produce contralateral paralysis of the tongue muscles as the descending corticobulbar fibers decussate to the nucleus of the hypoglossal nerve but without no muscle atrophy since the inferior motoneurons are intact.
The unilateral lesions are not typically a serious problem for patients, as the remaining hypoglossal nerve partly compensates any impediments.
The bilateral lesions can cause profound difficulty with speech and swallowing, as the patient cannot protrude the tongue for these necessary functions.
The study shows the origin of the sensory pathways (medial lemniscus, anterolateral system or spinal lemniscus), the origin and course of the motor pathways (corticospinal and corticobulbar tracts) and evidences that lesions of sensory and motor tracts induce clinical aspects related to the level at which the lesion occurs.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti
Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation. “The peer review process was efficient and constructive, and the editorial office provided excellent communication and support throughout. The journal ensures scientific rigor and high editorial standards, while also offering a smooth and timely publication process. We sincerely appreciate the work of the editorial team in facilitating the dissemination of innovative approaches such as the Bonori Method.” Best regards, Dr. Matteo Bonori.

I recommend without hesitation submitting relevant papers on medical decision making to the International Journal of Clinical Case Reports and Reviews. I am very grateful to the editorial staff. Maria Emerson was a pleasure to communicate with. The time from submission to publication was an extremely short 3 weeks. The editorial staff submitted the paper to three reviewers. Two of the reviewers commented positively on the value of publishing the paper. The editorial staff quickly recognized the third reviewer’s comments as an unjust attempt to reject the paper. I revised the paper as recommended by the first two reviewers.
