AUCTORES
Globalize your Research
Review Article | DOI: https://doi.org/10.31579/2690-4861/746
1 Department of Neurology, Hospital “Sant’Antonio Abate” Trapani, Italy.
2 Retired Professor of Human Anatomy. University of Palermo. Palermo, Italy.
3 Department of Medicine and Surgery of Acceptance and Emergency. Emergency Room (MCAU) of the Villa Sofia-Cervello Hospital in Palermo, Italy.
*Corresponding Author: Farina Elvira, Retired Professor of Human Anatomy. University of Palermo. Palermo, Italy.
Citation: Lipari Alessio, Farina Elvira, Lipari Luana, (2025), Somatotopic Organization of Cranial Nerves of the Medulla Oblongata: IX, X, XI, XII, International Journal of Clinical Case Reports and Reviews, 25(1); DOI:10.31579/2690-4861/746
Copyright: © 2025, Farina Elvira. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 07 March 2025 | Accepted: 24 March 2025 | Published: 04 April 2025
Keywords: nuclei of medulla oblongata; cranial nerves of medulla oblongata; somatotopy
Stemming from our previous studies regarding somatotopy of the spinal cord, oculomotor, trigeminal complexes and facial nuclei we examine the nuclei of origin of cranial nerve of the medulla oblongata to understand some neurological clinical and surgical aspects.
The cranial nerves, twelve pairs, emerge from inferior surface of the brain and are designed by a name and by Roman ordinal numerals (I-XII) from rostral position to caudal position. The cranial nerves differ from the spinal nerves; in fact, all spinal nerves are mixed, originating from the union of a ventral motor root and a dorsal sensory root, while the cranial nerves differ from each other in structure and function. The cranial nerves some are only motor, some only sensory and some are both sensory and motor. In relation to the parts of the brain the cranial nerves are divided into: telencephalic nerve, I or olfactory nerve, which originates from the olfactory neurons in the olfactory epithelium and extends to olfactory bulb; diencephalic nerve, II or optic nerve, which originates from ganglion neurons of the retina of the eye and extends to the lateral geniculate body; mesencephalic nerves III, IV, V; pontine nerves VI, V, VII, VIII; medullary nerves V, IX, X, XI, XII. An Overview of the cranial nerves was reported by Porras-Gallo and coll [1].
Galen (129—216 circa) was the first to distinguish nerves, tendons and ligaments and, also, to distinguish the “spinal nerves" originating from the spinal cord and "encephalic o cranial nerves" originating from the brain and indicates numerating seven pairs of brain nerves: 1, Optic; 2; Oculomotor; 3-4, Trigeminal; 5, Facial and Auditory; 6, Glossopharyngeal-Vagus-Accessory; 7, Hypoglossus.
Acuña and coll [2] report that Mondino de' Liuzzi (1265–1326) [3], Italian anatomist, wrote the first modern anatomical text Anothomia (1316) based on the dissection of human cadavers maintaining the classification of 7 pairs of cranial nerves in: I, optic; II, oculomotor; III, abducens; IV, trigeminal; V, facial; VI, vagus, VII, glossopharyngeal. Eustachio (1552) [5], Colombo (1559) [5] and Falloppio [6] indicated I nerve the optic nerve and II nerve the oculomotor nerve. that was differentiated in three nerves: oculomotor, abducens, trochlear, but Falloppio [6] indicated: the II pair the oculomotor nerve; the IV pair the abducens nerve, added a new VIII nerve the trochlear and, also, indicated the III cranial nerve (trigeminal) subdivided in three branches ophthalmic, superior maxillary and inferior maxillary or mandibular.
However, except for Thomas Willis’s [7] version, these proposals were unsuccessful. Bartholin Gaspar father (1611), as Porzionato [8] reports, introduced the nervus olfactorius as the I cranial nerve and Willis [7] in his Cerebri anatome (1664) was the first to list the olfactory tracts as the I pair cranial nerve and proposed a new classification composed of nine pairs cranial nerves; the I, II, III, IV, V, and VI cranial nerves were those we recognize today; Willis included VII pair the facial and vestibulocochlear nerves, VIII nerve encompassed the glossopharyngeal and vagus nerves, and the cranial root of the accessory nerve; the IX nerve consisted of the hypoglossal nerve and the spinal root of the accessory nerve [9]. The Willis’s formula by its popularity was consolidated and remained in place for more than 100 years until 1778, when Sömmerring [10] in his Doctoral Dissertation formulated the current classification composed of 12 cranial pairs although it during the nineteenth century undergoes many disputes.
The above-mentioned classifications of cranial nerves are represented in the following table.
| Nerves | Galen | Vesalius 1543 | Colombo 1559 | Falloppio 1561 | Willis 1664 | Bidloo 1685 | Sommering 1778 |
0/N(Nulla)/ I Terminal (near lamina terminalis) | Ayers H (1919) numbered it “cranial nerve I”, considering a total of 14 cranial nerves including also the septal—today vomeronasal—nerve. (Ayers: Vertebrate cephalogenesiss. IV. Transformation of the anterior end of the head, resulting in the formation of the nose. J Comp Neurol 30:322–342). Demsky and Schwanzel 1987 numbered it “cranial nerve 0” because it was rostral to the other twelve nerves. (Demsky LS and Schwanzel-Fukuda M: The terminal Nerve: Nervus Terminalis, Structure, Function and Evolution. Annals of the New York Academy of Sciences. Vol. 519). | ||||||
| I | X | X | X | X | Tract | Tract | Olfactory |
| II | 1° p. | 1° p. | 1° p. | 1° p. | 2° p. | 2° p. | Optic |
| III | 2° p. | 2° p. | 2° p. | 2° p. | 3° p. | 3° p. | Oculomotor |
| IV | X | X | 9° p. | 8° p. | 4° p. | 4°p. nerve pathetici | Trochlear |
| V | 3° p. 4° p. | 3° p. 4° p. | 3° p. 4° p. | 3° p. | 5° p. Trifacial | 5° p. nerve gustatorii | Trigeminus |
| VI | X | X | 8° p. | 4° p. | 6° p. | 6° p. Nervi timidi | Abducens |
| VII | 5° p. | 5° p. | 5° p. | 5° p. | 7° p. Facial (portio dura) acoustic (portio mollis). | 7° p. | Facial
|
| VIII | Vestibulo-cochlear | ||||||
| IX | 6° p. | 6° p. | 6° p. | 6° p. | 8° p. | 8° p. | Glosso-pharyngeal |
| X | 9° p. | Vagus | |||||
| XI | 10° p. | Spinal Accessory | |||||
| XII | 7° p. | 7° p. | 7° p. | 7° p. | 9° p. | 11° p. | Hypoglossal |
| I Cervical | 10° p. | ||||||
Table 1: Classifications of cranial nerve from Galen to Sömmerring
Recent literature data report some errors in the cranial nerves concerning their emergence and classification to be corrected. Corrales [11,12] evidenced that all illustrations of contemporary atlases analyzed characteristically show CNs VI, VII, and VIII all emerging from the pontomedullary groove as originally depicted by Sömmerring [12]. Differently the MRI findings show that the exit location of CN VI was caudal to the CN VII/VIII complex in 93% of cases and, also, multiple MR images analyzed coupled with microsurgical experience of this anatomical region indicate that CN VII enters the pons caudal to CN VIII. Revising a universally recognized numbering scheme would certainly create confusion, especially in transition. What is clear, however, is that future depictions of the anatomical arrangements of the brain stem exits of CNs VI–VIII ought to reflect accurate anatomy, even though it will remain at variance with the classic numbering system. Also, Prestigiacomo [13] reported other “errors” in medical illustration and medical nomenclature exist. It is well known that CNs I and II are actually tracts, in that they do not represent the first-order sensory neurons and have a different embryological origin. Nonetheless, they continue to be referred to as “nerves.”.
The nervus terminalis, nervus intermedius, and some AA might add the motor branch of CN V, although distinct, are not numbered separately.
The Sömmering classification [10], despite many disputes during the nineteenth century, has been accepted up to the present, recently Benninger and McNeil [14] proposed a new renumeration of cranial nerves following the application of the definition of a “cranial nerve”. To be defined as cranial nerve, the nerve must originate from brainstem and pass through a foramen skull. The nucleus of the cranial nerve contains secondary sensory neurons which synapse with ganglion protoneurons or motor neurons which reach the innervated muscles. These new criteria still produced 12 pairs of cranial nerve.
Current order (Sommering) | Assessment results (Benninger and Mc Neil) | New order (Benninger and McNeil) |
| (1) Olfactory | Eliminates 1) nucleus not in brainstem, (2) primary sensory neuron | |
| (2) Optic | Eliminated, (1) nucleus not in brainstem, (2) primary sensory neuron | |
| (3) Oculomotor | Becomes1st cranial nerve | (1) Oculomotor |
| (4) Trochlear | Becomes 2nd cranial nerve | (2) Trochlear |
| (5) Trigeminal | Is split into 2 separate nerves due to separate nuclei-current sensory remains as trigeminal with ophthalmic, maxillary, and mandibular divisions as 4th cranial nerve, motor of trigeminal becomes the masticatory nerve and is now the 3rd cranial nerve | (3) Masticatory (4) Trigeminal |
| (6) Abducens | Moves to the 5th cranial nerve | (5) Abducens |
| (7) Facial | Due to separate nuclei, facial be comes 6th cranial nerve; nervous intermedius becomes the 7th cranial nerve | (6) Facial (7) Nervous Intermedius |
| (8) Vestibulocochlear | Is split into 2 nerves due to separate nuclei and separate modalities. Vestibular nerve becomes the 8th cranial nerve; and the cochlear nerve becomes the 9th cranial nerve | (8) Vestibular (9) Cochlear |
| (9) Glossopharyngeal | Becomes the 10th cranial nerve | (10) Glossopharyngeal |
| (10) Vagus | Is split into 2 divisions due to target organs: (1) Laryngopalatopharyngeal (formerly cranial root of the accessory (XI) (2) Thoracoabdominal | (11) Vagus: (a) Laryngopalatopharyngeal (b) Thoracoabdominal |
| (11) Accessory | Eliminated, nucleus not in brainstem | |
| (12) Hypoglossal | Remains the same | (12) Hypoglossal |
Table 2: Renumeration of the cranial nerves by Benninger and McNeil [14].
The cranial nerves (III-XII) according to the Sömmerring classification [14] originate in brainstem from column, 1-6, (Figure. 1) that by subdivision and migration constitute the nuclei of origin of the cranial nerves (Figure. 2).
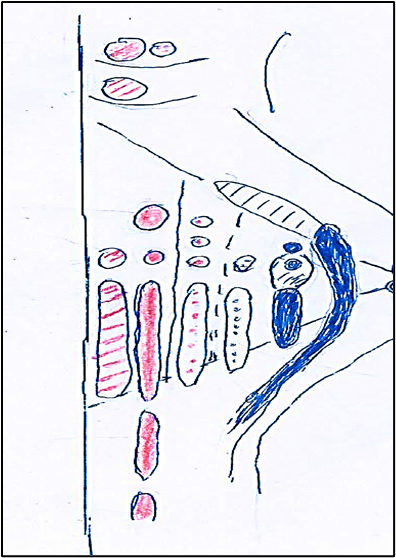
Figure 1: Columns (1-6) of the nuclei of the brainstem in medio-lateral direction. Col. 1 (red dotted lines). Column somatomotor that originates, caudo-rostrally, to somatomotor somitic nuclei: hypoglossal, abducens, trochlear, oculomotor. Col. 2 (colored in red). Column somatomotor that originates caudo-rostrally the somatomotor branchial nuclei: laryngeal, ambiguus, facial, masticatory. Col. 3 (red dots). Colomn visceromotor that originates caudo-rostrally to visceromotor nuclei: cardiopnemoenteric or ambiguus, inferior salivatory, superior salivatory, lacrimo-muconasal. Col.4 (blue dots). Column viscerosensory that, caudo-rostrally, originates the sensory nuclei: sensitive dorsal X, sensitive dorsal IX. Col. 5 (caudal blue colored; rostral dotted lines). Column viscerosensory that caudo-rostrally generates nuclei: solitary, inferior gustative, superior gustative. Col. 6 (caudal blue colored; rostral dotted lines) Column sensory that constitutesthe nucleus of the trigeminal nerve (V) which caudo-rostrally is divided into: caudal (blue) exteroceptive sensory part and rostral (dotted blue) proprioceptive sensory part.
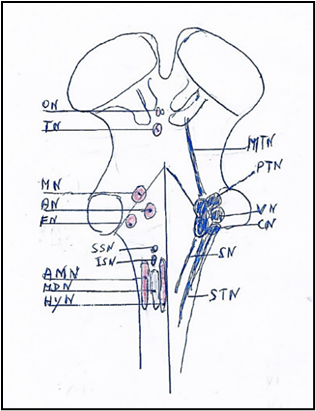
Figure 2. Brainstem nuclei with on left the motor nuclei and on right the sensory nuclei. The motor nuclei caudo-rostrally are: Hypoglossal nucleus (HyN). Motor dorsal nucleus (MDN). Ambiguus nucleus (AMN). Inferior salivatory nucleus (ISN). Superior salivatory nucleus (SSN). Facial nucleus (FN). Trochlear nucleus (TN). Oculomotor nucleus (ON). The sensory nuclei caudo-rostrally are: Spinal trigeminal nucleus (STN). Solitary nuclei (SN). Cochlear nuclei (CN). Vestibular nuclei (VN). Principal or pontine trigeminal nucleus (PTN). Mesencephalic trigeminal nucleus (MTN).
Somatotopy of Cranial Nerves: IX, X, XI, XII.
Previous our studies are regarding the somatotopy of the spinal cord [15], oculomotor complex [16], trigeminal complex [17]and facial nucleus [18], thus in this paper we report the somatotopic representation of the nuclei of the cranial nerves in the medulla oblongata: IX, X, XI, XII.
Glossopharyngeal Nerve and Nucleus
The glossopharyngeal nerve, IX cranial nerve, [19] is a “mixed nerve” with both motor and sensory fibers.
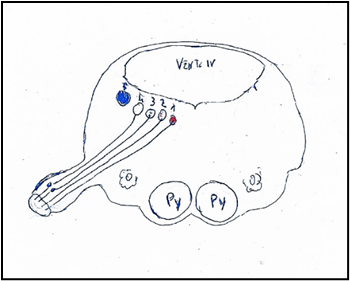
Figure 3: Nuclei of origin of the glossopharyngeal nerve mediolaterally. 1: Nucleus ambiguus. 2: Nucleus salivatory inferior. 3: Sensitive dorsal of glossopharyngeal nerve. 4: Solitary nuclei. Inferior gustative nucleus. 5: Sensory nucleus of trigeminal nerve. Py: Pyramid. O: Olive. VENT. IV: Ventricle IV.
The sensory fibers sensory convey information from the tympanic cavity, pharyngeal tympanic tube, fauces, palatine tonsils, rhino-pharynx, soft palate and the gustative fibers from 1/3 posterior or retrosulcal part of the tongue. The sensory fibers originate from the pseudounipolar neurons located in two ganglia outside the brainstem: superior ganglion or ganglion of Ehrenritter containing protoneurons which receive somatosensitive fibers or peripheral processes from auricle and send fibers to spinal trigeminal nucleus (tongue, pharynx); the inferior or petrosus ganglion or ganglion of Andersch containing the neurons: 1. neurons which receive viscerosensitive afferent fibers from fauces, carotid sinus, carotid glomus, tube and send fibers to the dorsal sensitive nucleus; 2. neurons which receive afferent taste fibers from the retrosulcal part of the tongue and project the fibers to rostral part of the nucleus of the solitary tract which is located dorsolaterally to the dorsal motor of vagus and anteriorly to the vestibular nuclei.
The motor fibers [20] originate from the cell bodies of the neurons located in the nucleus ambiguus which is situated beneath the floor of the IV ventricle, at level of the inferior olivary nucleus. The nucleus ambiguus is subdivided in the subnuclei with muscolotopic representation, so the laryngeal muscles are in caudal part of nucleus, the pharyngeal nuclei in the intermediate part, stylopharyngeus muscle and superior constrictor pharyngeal muscle in the cranial part. The glossopharyngeal nerve supplies the levator veli palatini muscle related the respiratory system, in particular with inspiration in rats [21] Recently, it was showed that the human pharyngeal muscles are heterogeneous in muscle fiber content [22] and that these fibers are arranged into two distinct and functional layers: a slow inner layer (SIL) and a fast outer layer (FOL) and that the glossopharyngeal (IX) nerveinnervates the slow inner layer, while the vagus nerve (X) innervates the fast outer layer. The two layers of fibers can be distinguished only in two-year old man, but they not found in the human newborns and nonhuman primates (monkey).
The efferent secretory parasympathetic fibers of IX nerve arise from the salivatory inferior nucleus, which is situated laterally and medially to rostral end of the ambiguus nucleus, between the hypoglossal nucleus medially and the nucleus of solitary tract laterally; these preganglionic parasympathetic fibers, by the own ramus, tympanic nerve, tympanic plexus and lesser superficial petrosal nerve arrive to “otic ganglion”. The “otic ganglion” gives origin to the postganglionic fiber which are associated with the “auriculotemporal nerve”, a trigeminal ramus, and terminate in the salivary parotid gland.
The visceral afferents of IX nerve arrive to solitary nucleus, together with the VII and X nerves.
Glossopharyngeal nerve has a communicating branch to the vagus nerve (CN X). Lesions to the CN IX are often accompanied by lesions to the CN X.
Caudal part of nucleus ambiguus gives rise to the cranial part of the spinal accessory nerve, while rostral part of this column gives rise to glossopharyngeal special visceral efferent fibers innervating the stylopharyngeus muscle.
The nerve vagus, X cranial nerve, [23] is mixed nerve containing both sensory or afferent and motor or efferent fibers innervating the larynx, pharynx and viscera (Figure. 4).
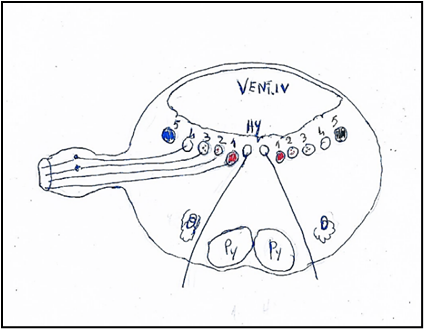
Figure 4: Nuclei of origin of the vagus nerve mediolaterally. 1. Nucleus ambiguus or Ventral nucleus of vagus nerve. 2. Nucleus cardiopneumoenteric or Dorsal nucleus of vagus nerve. 3. Sensory dorsal of vagus nerve. 4. Solitary nuclei. 5. Sensory nucleus of trigeminal nerve.
Hy: Hypoglossal nucleus. Py: Pyramid. O: Olive. VENT. IV: Ventricle IV.
The visceral afferent[24] fibers have the cell bodies located in the inferior or nodosus ganglion of vagal nerve, outside the brainstem, and arrive to the solitary nucleus and in the superior or jugular ganglion, outside the brainstem, and arrive to the trigeminal spinal nucleus.
The efferent fibers have own cell bodies in the nucleus ambiguus and the dorsal motor nucleus of the vagal nerve, both in the medulla oblongata.
The vagal fibers both afferent and efferent, are extensively distributed to the larynx, pharynx, trachea, lungs, heart, gastrointestinal tract (except the lower large intestine) pancreas, gallbladder, and liver.
The parasympathetic (dorsal motor nucleus) vagal nucleus is subdivided into nine subnuclei gathered in three regional zones: cranial, intermediary and caudal. The data in animalia suggest that the heart and lungs are represented in caudal sectors, the stomach and pancreas in the intermediate sectors, and restant abdominal viscera in the cranial and medial sectors.
The upper cervical esophagus is exerted on swallowing and peristalsis by somatic and visceral motoneurons, whereas the lower esophagus is exerted on only peristalsis by visceral motoneurons.
The results in rat [25] indicated that the upper cervical esophagus is innervated by the visceral medullary vagal motoneurons as well as the somatic spinal accessory motoneurons. The lower esophagus is innervated only by the visceral medullary vagal motoneurons.
The nucleus ambiguus (Fig 5) is the motor nucleus of both vagus and glossopharyngeal nerves. The nucleus ambiguus is located in the reticular formation about half way between the spinal trigeminal nucleus and inferior olivary complex, extends from the level of the decussation of medial lemniscus to levels through the rostral third of the inferior olivary complex; the fibers from nucleus arch dorsally, join efferent fibers from the dorsal motor nucleus of vagus nerve and emerge from lateral surface of medulla dorsal to the inferior olivary complex.
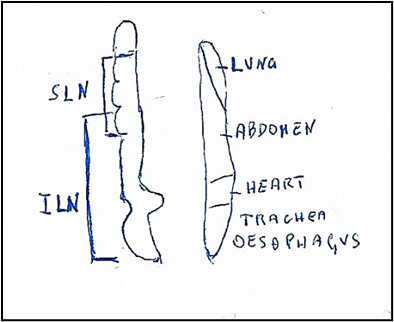
Figure 5: Nucleus ambiguus (On left). Somatotopic origin of laryngeal nerves. SLN: Superior laryngeal nerve innervating: upper oesophagyus, cricothyroid muscle, upper, middle, lower pharynx and soft palate. ILN: Inferior laryngeal nerve innervating the muscles: lateral cricoarythenoid, thyroarythenoid, interarythenoid and posterior crioarythenoid. Motot dorsal nucleus (On right). Viscerotopic localization of nuclei innervating: lung, abdomen, heart, trachea, oesophagus.
Caudal part of nucleus ambiguus gives rise to the cranial part of the spinal accessory nerve, while rostral part of this column gives rise to glossopharyngeal special visceral efferent fibers innervating the stylopharyngeus muscle.
The nucleus ambiguus in the rat [26] is made up of two major longitudinal divisions: a dorsal division, special visceral efferent component, comprising three rostrocaudally aligned subdivisions, and a ventral division, a general visceral efferent component, comprising at least two.
The dorsal division corresponds to the nucleus ambiguus in the narrow sense and comprises: a rostral compact formation part innervating the oesophagus, an intermediate semicompact formation innervating the pharyngeal and laryngeal muscles, and a caudal loose formation innervating the laryngeal muscles. Each of these formations displays a characteristic dendritic architecture. The stylopharyngeal and cricothyroid motoneurons are displaced rostral from the main pharyngeal and laryngeal motoneuronal pools. Thyropharyngeal (lower constrictor) motoneurons occupy the rostral half of the semi-compact formation and hypopharyngeal (middle constrictor) motoneurons its entire length.
The ventral division of the nucleus ambiguus corresponds to the external formation, extends along the entire length of the medulla oblongata, and contains preganglionic neurons innervating the heart and supradiaphragmatic structures innervated by the glossopharyngeal and the superior laryngeal nerves.
In the monkey [27] the nucleus ambiguus presents a column of neurons innervating the cricothyroid muscle extended from a level near the rostral end of the inferior olivary nucleus (IO) to a level caudal to its middle part. The cell columns of the cricoarytenoid, thyroarytenoid, lateral cricoarytenoid and inter-arytenoid muscles were located between the level rostral to the middle of the inferior olivary nucleus and that of the caudal end of the inferior olivary nucleus.
The nucleus ambiguus in a region of the nucleus located rostrally to the obex contains both populations overlap the motoneurons of the superior laryngeal nerve which innervates the cricothyroid muscle situated rostrally to the motoneurons of the inferior or recurrent laryngeal nerve which innervates other laryngeal muscles.
Recent studies of Pascual-Font and coll [28]. by neuroanatomical tracing study, shown in rats a more complex anatomical and functional organization; in the rat the recurrent laryngeal nerve does not contain any afferent axons from the larynx, in contrast to the pattern observed in many other species including man. The recurrent laryngeal nerve supplied only special visceromotor innervation to the intrinsic muscles of the larynx from motoneurons in the nucleus ambiguous. The superior laryngeal nerve contains afferent fibers which originate from larynx and reach the nucleus of the solitary tract.
The superior laryngeal nerve (SLN) also contained secretory efferent fibers originating from motoneurons in the dorsal motor nucleus of the vagal nerve, and special visceral efferent fibers originating from the ambiguus nucleus. Thus, this study shows that in the rat the innervation of the larynx differs in significant ways from that described in other species.
The accessory nerve [19] XI cranial nerve, emerges from the jugular foramen with glossopharyngeal and vagal nerves, but it does so by first ascending into the skull via the foramen magnum, and then exiting the central nervous system via the jugular foramen. Galen is the first to identify the accessory nerve including it with the vagal and glossopharyngeal nerves as his sixth pair. Willis [7], includes the hypoglossal nerve that is the IX cranial nerve and caudally includes the accessory nerve that is the X pair nerve referring to C1. Willis [7] excludes the accessory nerve from the encephalic nerves that is an irregular nerve because it originates entirely within the spinal cord. The Soemmerring classification [10] maintains the accessory nerve as cranial nerve XI without make sense that its nucleus and nerve begin more caudally than those of the hypoglossal or XII nerve. Benninger and McNeil [14] report that firstly Fredrici Arnold (1838) adds a cranial/bulbar root to accessory nerve and published a series of elaborately drawn anatomical plates depicting two components to the accessory nerve so that successively Henry Gray [29] in his first edition of Gray’s Anatomy reports two components of accessory nerve.
Recently Benninger and McNeil [14] believe that the structure known as the cranial root of the accessory nerve should not be included as part of the accessory nerve and it should be renamed. Furthermore, Benninger and McNeil [14] by applying contemporary embryological and anatomical findings, group the efferent peripheral nerves innervating the striated musculature into three groups: spinal, cranial and transitional. The efferent spinal nerves have a nucleus of origin within thespinal cord and innervate the musculature derived entirely from both somites and connective tissue that originates from mesoderm. The cranial nerves have nucleus in the brainstem and the same AA and also Galen, Willis and other AA propose a subdivision of efferent cranial nerves in three subcategories: 1) cranial somatic efferent (CSE) with target musculature derived from pre-otic somites (CSEpr), (oculomotor (III), trochlear (IV), and abducens (VI)); 2) cranial somatic efferent with target musculature derived from postotic somites (CSEpo) (vagus (X), Laryngopalato-pharyngeal motor (XI), and hypoglossal (XII)); 3) cranial branchial efferent (CBE) (trigeminal (V), facial (VII), glossopharyngeal (IX)), which have targeted musculature arising from somitomeres (non-somite paraxial mesoderm).
Benninger and McNeil (14) add to the categories of cranial and spinal nerves a third classification of peripheral nerve, a transitional somatic efferent (TSE) nerve, which represents the “accessory nerve proper” and combines characteristics of both cranial and spinal nerves (Table 1). Furthermore, same AA demonstrate Willis’s reasoning was correct affirming that the accessory nerve is “not an encephalic nerve” and should be regarded as a unique peripheral nerve.
Currently, the accessory nerve [30] is a only motor nerve (Fig 6) and comprises two components or parts: 1) a true cranial component, the true cranial nerve, which emerges from the medulla oblongata as a number of rootlets in series caudally with those of the vagal nerve; 2) a spinal component which arises as multiple rootlets from the anterior horn cells of C1-C5 spinal cord segments. These rootlets unite to form a single trunk which runs up within the vertebral canal and through the foramen magnum travels upward into the posterior cranial fossa and then leaves the skull through the jugular foramen and; just below the jugular foramen, the two components, cranial and spinal, separate.
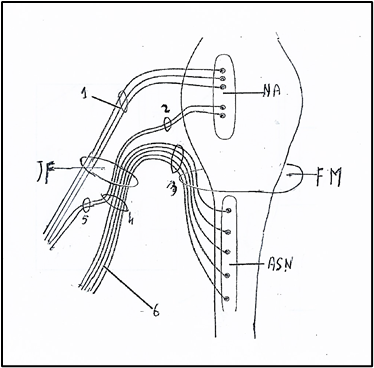
Figure 6. Nucleus ambiguus and nuclei of accessory nerve. NA: Nucleus ambiguus. ASN: Accessory spinal nucleus. 1: Nervus vagus. 2: Vagal root of accessory nerve; 3: Spinal root of accessory nerve. 4: Trunk of accessory nerve. 5: Internal branch of accessory nerve or vagal communicating branch to laryngeal muscles. 6: External branch of accessory nerve or spinal accessory nerve to trapezius and sternocleidomastoid muscles. JF: Jugular Foramen. FM: Foramen magnum. Figure modified from: Kierman J.A. Barr: Il sistema nervoso dell’uomo. A cura di Cocco L, Gaudio E, Manzoli L, Papa M. Edises p. 149. 2013.
The cranial component of the accessory nerve joins the vagus at the inferior vagal ganglion (a short distance below the jugular foramen) and thereafter ceases to have a separate identity. The cranial accessory fibers being entirely motor are distributed to the pharyngeal constrictor muscles via the pharyngeal branch of the vagus nerve.
The spinal component of the accessory nerve, SNA, runs postero-inferiorly to supply in the medial surface of the sternocleidomastoid muscle, gives ramifications to this muscle, crosses the posterior cervical triangle of the neck and enters in the inferior portion of the trapezius muscle.
The spinal component of the accessory nerve (Fig. 7) [31] comprises the group of the motoneurons supplying the sternocleidomastoid and trapezius muscles, the two occipital muscles which derivate from the lateral plate mesoderm, unlike the masticatory and facial muscles which derivate from both the cranial paraxial mesoderm and lateral plate mesoderm [32]. In rat the spinal accessory nucleus [31] longitudinally was found to be located in the caudal part (caudal 0.9-1.2 mm) of the
medulla oblongata, the whole lengths of cervical spinal cord segments C1-C5 and rostral fourth of C6. C1-C4) ([24]. In the caudal part of the medulla oblongata, the SNA was represented by a group of perikarya of motoneurons lying immediately ventrolateral to the pyramidal fibers that were passing dorsolaterally after their decussation. In the spinal cord, the motoneurons of the SNA were located in the dorsomedial and central columns at C1, in the dorsomedial, central and ventrolateral columns at C2 and in the ventrolateral column only at C3, C4, C5 and rostral quarter of C6. The perikarya of motoneurons supplying the sternocleidomastoid were located in the caudal part (caudal 0.9-1.2 mm) of the medulla oblongata ventrolateral to the pyramidal fibers that were passing dorsolaterally after their decussation. They were also located in the dorsomedial and central columns at C1, in the dorsomedial, central and ventrolateral columns at C2 and only in the ventrolateral column at the rostral three-quarters of C3. The perikarya of motoneurons supplying the trapezius muscle were located in the ventrolateral column only in the caudal three-quarters of C2, the whole lengths of C3, C4 and C5, and in the rostral quarter of C6.
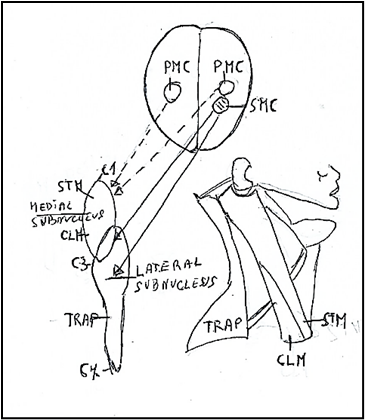
Figure 7: Subnuclei of the accessory nerve. Medial subnucleus extend between C1-C3 spinal segments innervating the muscles: sternomastoid muscle (STM) and Cleidomastoid CLM). Lateral subnucleus innervating the muscles: Cleidomastoid (CLM) and Trapezius (TRP). C1: First spinal segment. C3: Third spinal segment C7: Seventh spinal segment. PMC: Primary motor cortex. SMC: Supplementary motor cortex. Figure modified from Benninger and Mc Neil [14].
Besides, it is considered that the muscles of neck, trapezius and sternocleidomastoid, are transitional muscles [14] originated from mesoderm-derived striated muscle with connective tissue and osseous attachments that are neural crest born. These muscles are innervated by “transitional nerve” [14] having both cranial and spinal characteristics, but ultimately residing in the cervical spinal cord. Thus, the accessory nerve and its associated musculature should be regarded as a transitional nerve and transitional muscles, a new category of peripheral nerve and musculature.
The hypoglossal nerve, XII cranial nerve, [27] is a main motor nerve (Figure 8) innervating the somatic skeletal musculature of the tongue, but containing also afferent fibers.
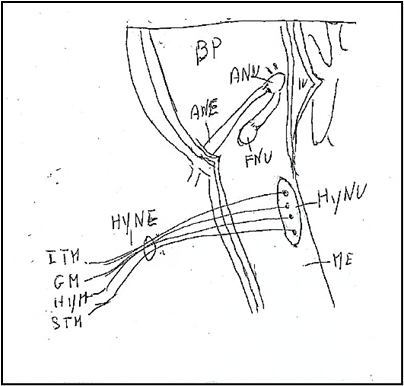
Figure 8: Hypoglossal nucleus and hypoglossal nerve.BP: Basilar Pons. ME: Medulla oblongata. IV: Ventricle IV. ANU: Abducens Nucleus. ANE: Abducens Nerve. FNU: Facial Nucleus. HyNU: Hypoglossal Nucleus. HyNE: Hypoglossal Nerve.. ITM: Intrinsic Tongue Muscles. GM: Genioglossus Muscle. HYM: Hypoglossus Muscle. STM: Styloglossus Muscle.
In cat [34] the primary afferent neurons or protoneurons of the hypoglossal nerve were located in the trigeminal ganglion, the superior ganglion of the glossopharyngeal and vagal nerves, and the first three cervical ganglia. The central projections of these protoneurons hypoglossal afferents were organized in a selective manner according to their cell origin: central projections of neurons in the trigeminal ganglion terminated in the subnuclei of the trigeminal nucleus: subnucleus dorsalis (Vpd) of the principal nucleus (Vp), lateral margin of the caudal pars interpolaris (Vi), interstitial nucleus and laminae I and V of the pars caudalis (Vc); 2. central projections of neurons in the superior ganglion of glossopharyngeal and vagal nerves terminated ipsilaterally in the caudal half of the solitary nucleus and bilaterally in the commissural nucleus [34] ;3. central projections of proprioceptive neurons in the C1-C3, first three cervical ganglia terminated in laminae I and V of the corresponding cervical cord segments [35].
In dog Chibuzo and Cummnings [36] found, by these intramuscular injections in the isolated extrinsic lingual muscles, that the primary neurons of the afferent fibers to lingual muscles, the genioglossus, hyoglossus, and styloglossus are located in the C1 spinal ganglion, proximal vagal (jugular) ganglion and trigeminal ganglion, ipsilaterally.
In monkey Kubota and coll. [37] found that the protoneurons situated in the C2, C3, C4 ganglia have the peripheral processes which, through a direct anastomosis “ansa hypoglossi or ansa cervicalis” between the cervical nerve I and the hypoglossal nerve, conduct the lingual proprioceptive impulses from the spindle muscle in tongue.
The hypoglossal nucleus originates the efferent fibers of the hypoglossal nerve. The hypoglossal nucleus forms a column of multipolar cells, about 18 mm long, that occupies the central gray of the medial eminence; it begins caudal to inferior olive and extends rostrally to region of the striae medullares of ventriculi quarti. Within the nucleus run the myelinated fibers which are the root fibers of the motor cells, the root fibers gather on the ventral surface of the nucleus, forming a series of rootlets [10-16] which pass ventrally, lateral to the medial lemniscus, and emerge on the surface of the medulla oblongata between the pyramid and inferior olivary complex.
The hypoglossal nucleus [20] is constituted of two nuclei posterior and anterior columns, each subdivided in medial and lateral subnuclei. The motoneurons have a myotopic organization, in particular the posterior subnuclei/posterolateral innervate the retrusor muscles (temporalis muscle) of the tongue; the subnuclei anterior/anteromedial innervate the protrusor muscles (medial or internal and lateral or external pterygoid muscles) of the tongue. Experimental data suggest that motoneurons of medial compartment innervate the transverse muscle (intrinsic muscles: transverse and vertical muscles and genioglossus muscle), while the motoneurons of the lateral compartment innervate the longitudinal muscles (styloglossus, hyoglossus, superior and inferior longitudinal muscles). (In proximity to hypoglossal nucleus are minor neuronal groups (perihypoglossal nuclei) that comprend the intercalatus nucleus (Staderini) with gustative and visceral connections, paramedian dorsal nucleus (reticular nucleus and the prepositus nucleus which is connected with the cerebellum and oculomotor nuclei.
In rat, the hypoglossal nucleus is subdivided in the dorsal and ventral parts. The dorsal hypoglossal nucleus [38] innervates the retrusor muscles and ulteriorly is subdivided in: a rostrolateral part with the neurons innervating the extrinsic, styloglossus muscle and a caudolateral part innervating the extrinsic hyoglossus muscle; the central and medial parts with the neurons innervating the intrinsic muscles, superior and inferior longitudinal muscles. The ventral hypoglossal nucleus [39] innervates the genioglossus muscle; this muscle in tongue of mammals has at least two subdivisions, one is horizontal and the other fans out obliquely; in dog, the hypoglossal nerve appears to have separate branches for each muscle subdivision. In rat the two subnuclei lateral and centrolateral of the ventral hypoglossal nucleus supply the two components of the genioglossus muscle.
In rat Mameli and coll [40]. showed that axons of neurons of hypoglossal nucleus also innervate the ipsilateral masseter muscle because they also spread into the masseter branch of the trigeminal nerve to target the polar portions of the masseter muscle spindles. Besides the retrograde double labelling, performed by injecting Dil into the pad and True Blue into the ipsilateral masseter muscle, showed labelled hypoglossal neurons in the medio-dorsal portion of the XII nucleus. The majority of these neurons were small (15-20micron diameter), showed fluorescence for Dil and projected to the mystacial pad. Other medium-size neurons (25micron diameter) were instead labelled with True Blue and projected to the masseter muscle. Finally, in the same area, other small hypoglossal neurons showed double labelling and projected to both structures.
A central mechanism [41] responsible for motor and premotor control of hypoglossal (XII) activity during swallowing activity, consists of the medullary and pontine populations of XII and trigeminal premotor neurons; in particular the Kolliker-Fuse nucleus has a prominent role in control of the inspiratory-related activity of the XII motoneurons supplying tongue protrusor and retrusor muscles.
In monkey macaca mulatta Morecraft and coll [42] evidenced that the hypoglossal nucleus receives inputs from extensive regions of cerebral cortex, specially from frontal, parietal cingulate and insula cortices. The hypoglossal nucleus received bilateral input from the face/head region of the primary (M1), ventrolateral pre- (LPMCv), supplementary (M2), rostral cingulate (M3), and caudal cingulate (M4) motor cortices. Additional bilateral corticohypoglossal projections were found from the dorsolateral premotor cortex (LPMCd), ventrolateral proisocortical motor area (ProM), ventrolateral primary somatosensory cortex (S1), rostral insula, and pregenual region of the anterior cingulate gyrus (areas 24/32). Dense terminal projections arose from the ventral region of M1, and moderate projections from LPMCv and rostral part of M2, with considerably fewer hypoglossal projections
Mameli and coll [43] found that the natural olfactory stimulation with amyl acetate significantly modulates the electrical activity of hypoglossal neurons and the electromyographic responses of the tongue musculature and provided the initial demonstration that olfactory information is conveyed from the olfactory bulb to the hypoglossal nucleus via the interpeduncular nucleus (IPn) by both fast disynaptic and different polysynaptic pathways. The latter, in particular, involve many of the brain structures that process olfactory information.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.
