AUCTORES
Globalize your Research
case report | DOI: https://doi.org/10.31579/2692-9406/128
1Department of Neurosurgery, University of Florida, Gainesville
*Corresponding Author: Brandon Lucke-Wold, Department of Neurosurgery, University of Florida, Gainesville
Citation: Yusuf Mehkri, Brandon McDonald, Sai Sriram, Ramya Reddy, Brandon Lucke-Wold. et al (2022) Recent Treatment Strategies in Alzheimer's Disease and Chronic Traumatic Encephalopathy. Biomedical Research and Clinical Reviews, 7(3); DOI: 10.31579/2692-9406/128
Copyright: © 2022 Brandon Lucke-Wold, This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 08 August 2022 | Accepted: 30 August 2022 | Published: 08 September 2022
Keywords: neurotrauma; alzheimer’s disease; chronic traumatic encephalopathy; pre-clinical models
Neurotrauma has been well linked to the progression of neurodegenerative disease. Much work has been done characterizing chronic traumatic encephalopathy, but less has been done regarding the contribution to Alzheimer’s Disease. This review focuses on AD and its association with neurotrauma. Emerging clinical trials are discussed as well as novel mechanisms. We then address how some of these mechanisms are shared with CTE and emerging pre-clinical studies. This paper is a user-friendly resource that summarizes the emerging findings and proposes further investigation into key areas of interest. It is intended to serve as a catalyst for both research teams and clinicians in the quest to improve effective treatment and diagnostic options.
Alzheimer’s Disease (AD) remains as the most prevalent cause of dementia worldwide and continues to be a significant health burden to society. Research in recent decades has significantly advanced our understanding of the pathophysiology of AD and its two neuropathological hallmarks: extracellular aggregations of insoluble forms of amyloid-β peptide (Aβ), known as Aβ or senile plaques, and intracellular neurofibrillary tangles (NFTs) predominantly comprised of hyperphosphorylated tau protein (p-tau). [1] First hypothesized by Hardy and Higgins in 1992, the long-standing amyloid cascade hypothesis (ACH) postulates that senile plaques are the causative agent of AD and the neurodegenerative sequelae that follow. 2 While this is the most favorable explanation for familial AD, studies have yielded conflicting results as to whether Aβ plaques are the initiator or consequence of sporadic AD, but it is generally agreed that they play an unequivocal role in both forms of AD.
Discoveries in AD research further elucidated the potential mechanisms behind other neurodegenerative diseases; namely, chronic traumatic encephalopathy (CTE). CTE is a sporadic tauopathy associated with repetitive minor head trauma and is most frequently seen in athletes of high-impact sports, such as boxing or American football, and victims of domestic violence. [3] While usually clinically indistinguishable, CTE and AD are neuropathologically distinct. CTE is characterized by perivascular p-tau deposition in the sulcal regions of the cerebral cortex, found irregularly within surrounding neuronal cell and cell process NFTs. [3] By contrast, AD demonstrates a progressive but specific distribution pattern of Aβ plaques and p-tau NFTs that begins in the transentorhinal region early on and spreads to virtually all isocortical association areas by the terminal stages. [4] This distinction led to the recognition of CTE as a separate diagnosis from AD in dementia patients, although both conditions may only be confirmed by post-mortem examination. [5,6]
Improvements in our understanding of the pathologic hallmarks of AD led to the development of multiple antibody drug therapies aimed at preventing or reducing Aβ plaque load in the brain, which have proven to be largely ineffective in altering the course of AD. [7,8] Like other early models for monoclonal antibody drugs, solanezumab targeted soluble forms of Aβ, which proved ineffective in two phase III trials. [8] A phase III clinical trial for gantenerumab, a human monoclonal antibody that instead binds to aggregated forms Aβ and removes plaques via Fc-receptor phagocytosis, did not demonstrate efficacy either and was terminated prematurely for futility.9 Based on compiled statistical data from clinical trials, the most recent evidence suggests that a general reduction in Aβ plaques does not significantly improve cognition, suggesting that Aβ alone may not be the right target. [10] Or, as some newer articles theorize, we may be targeting the wrong hormetic form of Aβ. [11]
Perhaps the most controversial drug is aducanumab, a monoclonal antibody targeting aggregated forms of both soluble and insoluble Aβ. The drug received accelerated FDA approval for the treatment of AD in 2021[12]. despite conflicting results from its two phase III clinical trials and lack of established correlation between reduced plaque load and cognitive improvement[13]. The decision elicited a substantial outcry from the research community, and a case for the accelerated withdrawal of aducanumab was published by early 2022[14]. Despite its controversy, the drug was found to have a downstream effect on p-tau levels in cerebrospinal fluid (CSF), which has major implications in the treatment of other neurodegenerative disorders aberrantly expressing p-tau, such as CTE [15,16]. These developments have led researchers to explore tau hyperphosphorylation and other biochemical mechanisms as potential targets for drug therapies in AD, CTE, and related neurodegenerative disorders.
Emerging clinical trials in Alzheimer’s Disease
Several emerging treatment targets for AD are currently under early investigation in clinical trials. Two central themes in AD pathology are amyloid aggregation and neuroinflammation, which together cause dysregulation of homeostasis and establish a neurotoxic environment. Here, we highlight some recent advances in the targeting of these two domains.
Targeting amyloid pathways
Amyloid-β oligomer (AβO) deposition is thought to promote neuronal death by disrupting mitochondrial and lysosomal membrane integrity,[17,18]. causing endoplasmic reticulum dysfunction, [18,19]. inducing a high intracellular calcium state, [20]. and promoting pro-inflammatory oxidative stress [21]. Targeting this amyloid pathway may, in turn, confer a neuroprotective benefit in AD patients. To this end, CT1812 is a small molecule sigma-2 receptor antagonist which is recently under investigation in a phase 2 trial for its role in synaptic AβO clearance [NCT03507790]. In preclinical studies, though treating cultured neurons with human AD-derived AβO led to vesicle trafficking deficits, these deficits were abolished when cultures were pretreated with CT1812.22 In addition, CT1812 improved synaptic density in neuronal mouse cultures by displacing AβO from receptors, facilitated AβO clearance into CSF in vivo mouse models, and improved cognitive performance in a mouse model of AD [22]. Promising data from a phase 1b trial of 19 patients receiving placebo or CT1812 for 28 days revealed improved AβO clearance into the CSF of patients receiving CT1812 [22]. However, higher-powered studies with longer treatment durations focusing on cognitive outcomes are necessary and are already underway.
Other variants of Aβ have recently come under interest as treatment targets. In particular, amyloid precursor protein (APP) can be cut by secretases and modified by glutaminyl cyclase to form pyroglutamylated forms of Aβ (pE-Aβ), a particularly cytotoxic variant of Aβ known to induce synaptic dysregulation [23,24]. Recently, the small molecule glutaminyl cyclase inhibitor PQ912 has been explored as a potential treatment of AD. One preclinical study of a transgenic AD mouse model treated with PQ912 displayed promising results, both decreasing pE-Aβ in vivo and improving cognitive performance [25]. Another recent phase 2 study of PQ912 of 120 patients randomized to PQ912 or placebo groups reported no significant adverse events (AE) for the PQ912 group, a significant reduction in glutaminyl cyclase activity, and a reduction in the glial activation marker YKL-40 [24]. Notably, though PQ912 was associated with improved performance on the one-back cognitive test, there were no differences between PQ912 and placebo groups in the mini-mental state examination (MMSE) scores [24]. A phase 2A study for use of PQ912 to assess cognitive function in early AD patients is underway [NCT03919162].
A novel proteopathy has been recently implicated in the progression of AD. Filamin A (FLNA) is a scaffolding protein thought to be altered in AD. Altered FLNA allows for Aβ and nAChR-mediated neurofibrillary tangle formation with activation of TLR4 and subsequent neuroinflammatory marker release [26]. PTI-125 is a small molecule which resets the altered variant of FLNA back to normal [26] and, in a transgenic AD mouse model, improved certain cognitive performance measures, reduced neuroinflammation, and decreased other biomarkers of AD progression [27]. In a recent phase 2a study consisting of 13 AD patients treated with 28 days of PTI-125, no drug related AE were observed [28]. Treatment with PTI-125 was appropriately associated with the reduced presence of altered conformation FLNA. Importantly, markers of AD progression including tau, neuroinflammatory cytokines such as IL-1B, IL-6, and TNFa, neurogranin, and neurofilament light chain were significantly reduced following 28 days of PTI-125 treatment in this study [28]. Though cognition was not evaluated in this study, a current phase 2b study seeks to add to previous work by randomizing a larger number of participants, assessing CSF markers of AD progression, and evaluating cognition [NCT04388254].
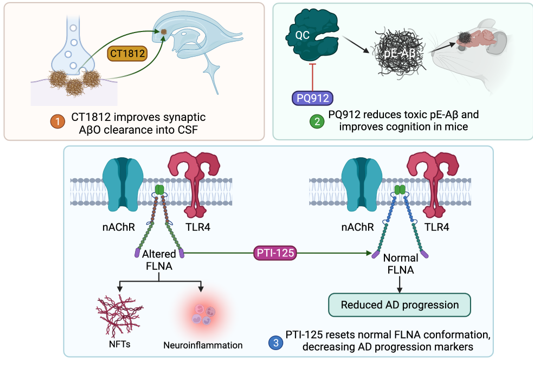
Figure 1: Molecular pathways of amyloid-targeting emerging therapeutics. Abbreviations: Aβ, amyloid β; pE-Aβ, pyroglutamylated forms of Aβ; QC, glutaminyl cyclase; TLR4, toll-like receptor-4; nAChR, nicotinic acetylcholine receptor; FLNA, filamin A; NFT, neurofibrillary tangle
Targeting neuroinflammatory pathways
Neuroinflammatory pathways have become an important drug target due to their prevalent role in the progression of AD [29]. One hallmark of AD is an elevated burden of fatty acid oxidation and synthesis. While fatty acids are crucial for proper brain and neuron function, high levels of fatty acid oxidation both modulates inflammatory processes and promotes neurodegenerative mechanisms [30,31]. CMS121 is a small molecule fatty acid synthase inhibitor which reduces oxidative damage by preventing excess lipid peroxidation [31]. Administration of CMS121 in neuronal and microglial cells as well as a transgenic AD mouse model led to decreased markers of lipid peroxidation and neuroinflammation. Spatial learning and memory tests of APPswe/ PS1ΔE9 transgenic mouse models treated with CMS121 also revealed improved cognitive function compared to wild type mice [31]. A phase 1 trial is currently underway to evaluate the long-term safety of this drug in healthy patients [NCT05318040].
AD has an impact on multiple pathways in the immune response. Therefore, treatments targeting the immune response from multiple angles may represent a more promising immunomodulatory approach. Recently, the antineoplastic TNFa inhibitor lenalidomide has come under interest for the treatment of AD due to its pleiotropic effects of modulating both innate and adaptive immune responses [32,33]. Notably, lenalidomide simultaneously lowers the expression of pro-inflammatory agents (TNFα, IL-6, and IL-8) and increases the expression of anti-inflammatory cytokines (IL-10) [33]. Preliminary results in vivo transgenic AD mouse models treated with lenalidomide showed significant decreases of key markers of AD progression including BACE1 and Aβ plaques as well as pro-inflammatory markers such as TNFa [33]. Despite this, special care should be exercised in further work considering some reports of lenalidomide-induced cognitive impairment [34]. A phase 2 trial is currently in progress to determine the efficacy of lenalidomide in reversing cognitive impairment in patients with mild to moderate AD [NCT04032626].
Despite the predominant view that neuroinflammation leads to progression of AD, recent work has investigated the pro-inflammatory cytokine granulocyte macrophage colony-stimulating factor (GM-CSF) as a potential treatment for AD. Interestingly, studies suggest that individuals with autoimmune diseases like rheumatoid arthritis (RA) have a lower risk of developing AD, [35] and GM-CSF has been shown to be elevated in patients with RA [36]. Sargramostim is an FDA approved recombinant human GM-CSF, and efforts have been made to determine the efficacy of this drug for the treatment of AD. Preclinical data of transgenic AD mouse models treated with sargramostim show improved cognitive function and increased activated microglia [37,38]. One phase 2 trial of 40 patients receiving either sargramostim or placebo for 3 weeks displayed its immunomodulatory effects, with upregulation of IL-6, IL-10, and TNFa, and downregulation of IL-8. Importantly, a statistically significant cognitive improvement was seen in the MMSE [37]. A safety analysis conducted in this study did not display any serious AE or amyloid changes on imaging [37]. Despite this, future clinical studies are underway to determine the long-term safety and tolerance of the drug on patients with mild to moderate AD [NCT04902703].
Alzheimer's: Preclinical studies and potential pathways warranting further investigation.
Alzheimer’s disease (AD) is biologically marked by β-amyloid plaques and Tau neurofibrillary tangles [39]. As such, efforts till now have largely targeted amyloid [40]. Other major targets, according to recent Phase 2 and 3 clinical trial therapies, focus on amyloid, synaptic plasticity/neuroprotection, Tau tangles, and neuroinflammation [41]. However, anti-amyloid strategies have been largely disappointing [40]. Several other pathways have come to light recently, warranting further investigation. These pathways include but are not limited to neuroinflammation, [42] oxidative stress, [43,44]. efects in mitochondrial dynamics and function, [45]. cholesterol and fatty acid metabolism, glucose energetic pathways impairments in the brain, [46,47] autophagy failure, [48] and others [49,50]. The following paragraphs will describe major pathways with broad highlights, in hopes of pointing out potential therapies in pre-clinical models of Alzheimer’s disease.
Inflammation, especially in the central nervous system, is an acute process that physiologically provides protection against infection, toxins, and injury [40,49]. However, when the balance between pro- and anti-inflammatory signals is disrupted, this can lead to chronic neuroinflammation, [51,53] which has been strongly linked to AD [54,55]. In a pro-inflammatory state, the increased levels of proinflammatory cytokines can cause mitochondrial stress via Aβ signaling as well as increase oxidative stress and subsequently, blood-brain barrier (BBB) permeability [56,57]. Moreover, a major pro-inflammatory cytokine, IL-18, leads to increased Cdk5 and GSK-3β, both of which are key players in Tau hyperphosphorylation [57]. While neuroinflammation may not trigger AD onset, it seems to play a significant role in exacerbating AD progression and/or symptoms via Aβ and Tau pathologies [58]. Recent pre-clinical studies have investigated curcumin, a polyphenol fund in turmeric, to be potently antioxidant and anti-inflammatory [59]. Further investigation is ongoing in terms of its role with regards to AD pathogenesis [59]. Another physiologic system, that if overactivated, can lead to AD symptoms is the complement system [60]. An overactive complement system influences Aβ, Tau, and APOE4, which can lead to AD progression [61,62]. Both pathways are propagated by non-neuronal supportive cells, which normally protect neurotransmission. However, in the setting of inflammatory cytokines (e.g.: IL-1β and TNF-α), the physiologic protection of neurotransmission is inhibited, leading to AD-related symptoms [52,63].
Another major focus of pre-clinical research is oxidative stress (OS), which has been linked to the prodromal phase of AD and not necessarily AD onset [64,65]. Increased OS, due to mitochondrial dysfunction, energy metabolism imbalances, or natural aging, can impair several key neuronal physiologic functions – namely, neuronal plasticity, cytoskeletal structure, and cellular communication [49,66-69]. Neurons, in specific, are quite sensitive to OS since the normal antioxidant is low [70]. Increased levels of reactive oxygen species (ROS) and reactive nitrogen species (RNS) leads to Tau phosphorylation and eventual destabilization of microtubules. This causes a major disruption in neuron polarity and intracellular trafficking, leading to decreased function of synapses [Munoz 32433996]. [66] In addition, Aβ is also tied to ROS and RNS and other mediators of OS (e.g.: NOX, TGF-β, NF-kβ, Nrf2). [64,71,72] Excess ROS causes oxidization of nucleic acids (which leads to lethal damage and promotes protein aggregation) as seen in post-mortem reports of AD patients [73]. OS is also tied to several other pathways and thus, is an area of further investigation.
One area that OS is linked to is glucose hypometabolism. High ROS levels reduces the concentration/availability of enzymes in the glycolytic cascade, leading to glucose hypometabolism [74-76]. In AD, this is confirmed via intracellular lesions in a subset of neurons with the highest metabolic requirements, that morphologically have elongated, thin axons and reduced or absent myelin sheaths [77]. Insulin signaling is another area of significant interest due to its association of AD, as Aβ oligomers bind insulin receptors, causing internalization of them [78-80]. The association of insulin signaling to AD is further supported by observing increased AD risk in populations affected by insulin-related comorbidities (e.g.: diabetes mellitus) [81]. Ultimately, altered insulin signaling can lead to neuroinflammation, which is tied to increased levels of Aβ and GSK3β, leading to Tau hyperphosphorylation, as previously mentioned [80,82,83]. Thus, insulin signaling is another area that warrants further investigation with relation to AD.
Furthermore, vascular dysfunction or cerebrovascular abnormalities are common comorbidities noted with AD, although the precise correlation with onset and/or progression of AD symptoms needs to be further elucidated [84]. The general principle is thought to stem from the altered homeostasis that arises from disrupted blood flow and/or pressure. This can lead to microfractures in the BBB also, which lends itself to neuroinflammation risk and eventual AD-related symptoms as previously mentioned [47,85-87]. In AD specifically, animal models link ROS to causing increased levels of advanced glycation end products (AGE) and their receptors (RAGE) in vasculature, which is linked to Aβ plaques. This is an active area of research, with drugs targeting AGE and/or RAGE being tested in clinical trials [41,88-91]. RAGE is also categorized as a pattern recognition receptor and is tied to innate immunity and inflammation. Lastly, increased levels of AGE and RAGE are tied to AD but are also found due to natural aging. This provides another clue as to why aging is a risk factor for AD [88,89,92].
Lastly, impaired autophagy has been linked to AD, based on animal models and AD patients [64]. APOE4 overexpression leading to Aβ accumulation within lysosomes causes neuronal death in the hippocampus, especially CA2/3 regions in animal models [93,94]. Furthermore, high levels of ROS inhibit autophagosome fusion with lysosomes [64]. These two pathways link impaired autophagy to AD. As such, current preclinical studies are further looking into restoring physiologic autophagosome clearance to curb the progression of cognitive symptoms [95]. All in all, AD pre-clinical research spans a wide array of topics, including altered neurotransmission and more, displaying the complex, multifactorial pathogenesis of AD, lending itself to multiple potential therapeutic targets in the future.
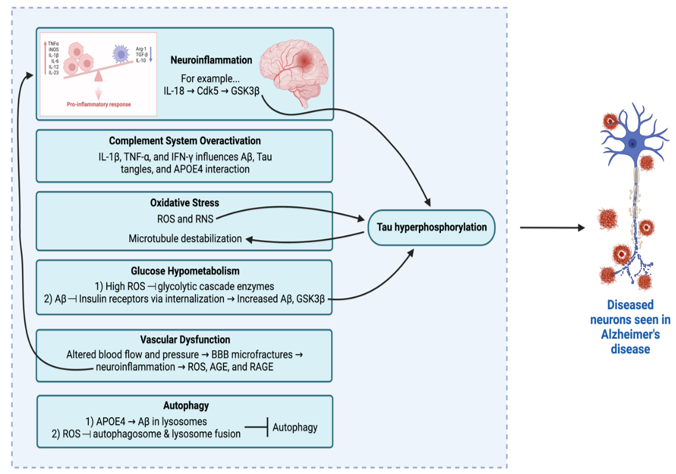
Figure 2: Summary of Select Pre-Clinical Alzheimer’s Disease Pathways
Diagnostic and Management Considerations
Chronic Traumatic Encephalopathy (CTE) is a neurodegenerative condition characterized by an accumulation of hyperphosphorylated tau protein depositions throughout the brain.96 Microscopic and gross neuropathological changes originate from exposure to repetitive head impacts, leading to a progression of cognitive and neurobehavioral deficits [97]. Due to the unique neuropathological phenotype associated with CTE, confirmational diagnostic strategies currently require post-mortem examination [98,99]. Overall, these diagnostic limitations present challenges for adequate management and therapeutic intervention. Thus, there is a critical need to improve diagnostic strategies through comprehensive psychiatric guidelines, imaging modalities, and biomarker analysis (Figure 3).
Neuropsychiatric Diagnosis and Symptomatic Management
Psychiatric evaluation of neurodegenerative diseases offers valuable preliminary diagnostic guidance. Previous studies have examined clinical features associated with CTE symptomatology to identify criteria correlated with cognitive and behavioral dysfunction [100,101]. Clinical guidelines were established in 2014 for diagnosis of Traumatic Encephalopathy Syndrome (TES), used as a proxy to describe the clinical symptoms of CTE [102]. Patients with TES present with progressive, cognitive deficits and/or neurobehavioral dysfunction associated with a prior history of repetitive head impacts [103]. Past exposure to repeated head trauma is of particular significance and the association between repeated mild TBI and neurodegenerative diseases is currently being investigated [NCT04124029]. Neurological deficits associated with TES can be classified into four symptomatic categories, including: cognitive, behavior, mood, and motor deficits [104]. Clinical assessments for these sequalae include evaluations for memory impairment and executive functioning, aggression and depression, and ataxia and dysarthria, respectively [100,105]. TES guidelines were validated for diagnostic efficacy in 2021, and researchers determined only cognitive symptoms were significantly associated with CTE [106]. However, these comprehensive criteria do provide useful methodology for excluding other neurological disorders and have been beneficial in establishing relevant management protocols. Current management strategies involve various forms of clinical intervention, including cognitive rehabilitation, behavioral therapy, as well as vestibular and ocular therapy [104]. Pharmacological agents have also been used for managing CTE, including stimulants, dopamine agonists, selective-serotonin reuptake inhibitors (SSRI’s [104]. These current management strategies have shown success for alleviating the cognitive and neurobehavioral deficits experienced clinically, however, none have shown efficacy for treating the disease. Thus, current limitations associated with TES guidelines must be overcome to ensure the development of effective therapeutic strategies.
Diagnostic Imaging Modalities
Several imaging modalities have been utilized to assess the diagnostic efficacy of examining neuropathological features associated with CTE. T1- and T2-weighted magnetic resonance imaging (MRI) are particularly useful in identifying gross structural changes associated with the disease [107]. Indeed, structural MRI indicated ventricular enlargement, cavum septum pellucidum and cortical atrophy were all significantly correlated with CTE [108]. Cortical atrophy was also shown to be correlated with AT8-immunostaining for tau pathology. Diagnostic efficacy for structural MRI is currently being investigated to determine how neurostructural changes are associated with chronic neurological outcome [NCT05235802]. In contrast, diffusion tensor imaging (DTI) may provide unique insight into identifying axonal integrity, and the dysfunction associated with repeated head impacts, specifically diffuse axonal injury (DAI) [109]. Using fractional anisotropy as a metric for evaluating axonal integrity, previous DTI studies showed that increased axonal disruption is associated with regions of hyperphosphorylated tau deposits [110]. Previous studies have also used imaging modalities to identify changes in metabolic profiles. Results from magnetic resonance spectroscopy (MRS) concluded metabolic changes in the anterior cingulate gyrus; including glutamate, glutathione, and myo-inositol, were all significantly correlated with behavior and mood domains [111]. Positron emission tomography (PET) has also been studied for confirming CTE diagnosis due to the molecular specificity associated with ligand binding. Former professional National Football League players were shown to have increased uptake in Flortaucipir in bilateral superior frontal, bilateral medial temporal and left parietal regions when compared against controls [100]. However, uptake was not significantly correlated with neuropsychiatric examinations. Other imaging molecules, including AV1451 and T807 have been validated for diagnosing CTE, but with variable success [112,113]. Currently, PET analysis using AV1451 is being investigation through the DIAGNOSE-CTE Research project, to be completed in 2023 [NCT02798185] [114].
Pathophysiological and Genetic Biomarkers
Fluid biomarkers, through collection of saliva, blood, and cerebrospinal fluid (CSF), have also been examined in patients suspected of CTE to assess diagnostic efficacy. Biomarkers associated with axonal injury, including tau, and neurofilament proteins light and heavy (NfL and NfH) have each been widely studied and associated with CTE [115]. Additionally, NfL has shown to be correlated with fractional anisotropy measurements from DTI studies, providing further rationale for usage in CTE diagnostics [116]. Other biomarkers including glial acidic fibrillary protein (GFAP) and s100b have also shown promise for validating pathophysiological features associated with CTE, specifically astrogliosis [117]. However, phosphorylated tau and amyloid beta remain primary diagnostic markers for discerning between CTE and AD [6]. Validation of fluid biomarkers is still an area of active investigation and current clinical trials are determining the proteomic and transcriptomic profiles associated with CTE [NCT04928534]. Additionally, genetic markers, including APOEε4 and TMEM106B may also be useful for diagnosing CTE, specifically for establishing links between genetic predispositions and CTE [118,119]. However, pathophysiologic, and genetic biomarkers alone do not possess adequate power for definitively diagnosing CTE. Thus, there remains a critical need to optimize and validate effective diagnostic and therapeutic protocols through pre-clinical studies.

Figure 3: Current diagnostic and management considerations for CTE. Cognitive and behavioral dysregulation associated with CTE was used to establish neuropsychiatric criteria for diagnosing TES, and symptomatic management for TES includes clinical and pharmacological intervention. Additionally, previous studies have validated the diagnostic efficacy for both imaging modalities and biomarkers and these strategies are currently under investigation in active clinical trials.
Preclinical Models
The development of preclinical animal models of CTE is challenging but necessary for the further investigation between the pathophysiological link between repetitive head trauma and chronic neurodegenerative tauopathy. While there are many different preclinical models of TBI, only a small subset has utilized repetitive and often mild injury to characterize chronic timepoints post-injury where the development of tauopathy is predicted to occur in CTE. Preclinical models of repetitive mild TBI (rmTBI) to investigate CTE are also a relatively recent development, with most experimental models being created in the past fifteen years. The experimental models that have investigated tau pathology following rmTBI have mainly been conducted in rodents utilizing diverse brain injury induction methods with or without genetic modifications in the tau gene, transgene expression of human tau (hTau), or overexpression of tau. Brain injury induction methods in rodent CTE models include both diffuse injuries, such as the fluid percussion injury (FPI), rotational injury to include the closed-head impact model of rotational acceleration (CHIMERA), and exposure to blast, and more focal brain injuries such as closed head injury (CHI), controlled cortical impact (CCI), or weight-drop models.
Diverse findings regarding rmTBI and tau pathology have been reported in preclinical models. Previous literature reviews have extensively covered preclinical CTE model findings from the years 2019 and prior [120-124]. Here, we have summarized here additional findings in CTE preclinical rodent models from 2020 to the present in Table 1. It is of interest to note that there is still conflicting evidence regarding presence and pathological severity of tauopathy following rmTBI among preclinical studies, with some studies identifying neuronal tau accumulation and others finding no differences in tau pathology. These differences are likely due to comparisons between models of different TBI injury paradigms and differences in TBI severity, timeline of repetitive injuries, and selected endpoints.
In modeling rmTBI with the goal of understanding the etiological link to CTE, there should be enhanced care taken in the field regarding consistency of parameters between models and a more stringent focus on translational capabilities of specific models. For example, rodent modeling of repeated rotational TBI may not be optimal in recapitulating the same forces and outcomes as human rotational brain injury due to the size of the rodent brain and lack of gyri and sulci [126,127], which are important not only in altering the way physical forces from the injury are distributed across the brain, but sulci and associated gray-white matter ratios are also highly associated with tauopathy in human patients [127,128]. Another important point in preclinical modeling of CTE is translatability in choice of brain injury induction method. Recent evidence has demonstrated that the risk of developing CTE is more associated with impact TBI, such as concussion events experienced in sports, versus blast TBI as experienced due to occupational exposure within the military [129]. Therefore, it may be critical to choose brain injury induction methods more associated with CTE like focal brain injury. Translation of preclinical research findings to better understanding of human CTE may also mean expanding the typical CTE model to new species whose brains are larger, gyrencephalic, and/or protected by a skull whose anatomy is more similar to humans [130,131]. Additional parameters to carefully consider in modeling CTE is the spacing of rTBI injuries, the age of the rodent during rmTBI, and the endpoint(s) at which pathology is assessed [102,122,132,133]. To better replicate the human timeline of rTBI, injuries should be spaced over a long period of time during adolescence to young adulthood, and chronic endpoints should be used for evaluation of CTE pathology. Currently, most models of rmTBI and CTE utilize short spacing of injuries and endpoints in rodents are typically chosen at 1-1.5 years of age, which only equates to mid-life in humans [134]. Overall, while preclinical studies of CTE represent one of the most favorable methods by which to investigate the pathophysiological link between rmTBI and CTE, there are key avenues to be addressed to enhance the translational capacity of preclinical models and enhance the investigation of causative mechanisms of CTE.
| Study | Animal | rTBI model | Injury Paradigm | Age | Tau Detection | Endpoint(s) | Findings |
| Tadepalli et al. 2020 [135] | Wild-type (WT) male rats | Mild weight drop (mWD) | Single mild (mTBI), re- petitive mild (rmTBI – 5 hits, 24 h apart), rapid repetitive mild (rapTBI – 5 hits, 5 min apart) or a single severe (sTBI) TBI | 8-10 months | Enzyme-linked immunosorbent assay (ELISA) | 8 weeks post-injury (PI) | No differences in phosphorylated tau (pTau); rmTBI group decreased performance in Novel Object Recognition (NOR) test |
| Cheng et al. 2020 [136] | Tau-overexpressing male mice (Tau58.4) | rmTBI via closed head impact (CHI) | 42 impact (6 impacts/day for 7 days) | 3-4 months | Immunohistochemistry (IHC) | 1 month PI | No differences in pTau, oligomeric Tau; rmTBI Tau-overexpressing mice had enhanced gliosis and peripheral immune cell infiltration |
| Bachstetter et al. 2020 [137] | WT or rTg4510 tauopathy male mice | CHI | Single or 2nd CHI exposured 1 day or 7 days PI | 4 months (WT), 2 months (rTg4510) | ELISA, Western Blot (WB) | 7 days PI (WT), 13 days PI (rTg4510) | Increased pTau and total Tau in 2 hit WT CHI mice; increased pS396/S404 positive tau in 1 and 2 hit rTg4510 CHI mice, no difference in total tau |
| Niziolek et al. 2020 [138] | Male mice with or without either i. pharmacological depletion of acid sphingomyelinase (Asm) or ii. genetic ablation of Asm (Asm-/-) | Moderate WD | Single | 8-10 weeks | IHC | 1 day or 30 days PI | TBI increased hippocampal pTau at 30 days PI and this accumulation is partially prevented by Asm inhibition |
| Tang et al. 2020 [139] | WT or transgenic Tau male mice (P301S) with or without Fyn kinase inhibition | CHI with or without chronic variable stress (CVS) | 14 hit (1 per day) over alternating hemisphere | 2 months | IHC | 5.5 months PI | Injury increased pTau but not total tau; Fyn inhibition rescued pTau levels to Sham |
| Angoa-Perez et al. 2020 [140] | WT male mice | WD | Exposed to a total of 20 head impacts (1 per day for 5 days [Monday-Friday with weekends off]) | 8 weeks | IHC | 0, 45, or 90 days PI | Increased pTau at 90 days PI |
| Bugay et al. 2020 [141] | WT male mice | Blast | 3 exposures, 1 per day over 3 consecutive days | 10 weeks | WB | 7 days PI | Increased tau and pTau in rmTBI group |
| Ojo et al. 2020 & Eisenbaum et al. [142,143]. | hTau mice | CHI | 2 injuries/week for 3 months | 3 months | Ex vivo tau uptake by cerebrovascular cells | 3, 6, or 12 months PI | Decreased tau uptake in rmTBI mice |
| Garcia et al. 2021144 | Male rats | Blast | 3 exposures, 1/day for 3 consecutive days | 10 weeks | WB | 10 days PI | Elevated pTau in blast animals and correlating neurobehavioral deficits |
| Dickstein et al. 2021 [112] | Male rats | Blast | 3 exposures, 1/day for 3 consecutive days | 10 weeks | WB, IHC | 6 weeks or 10 months PI | Elevated pTau at both endpoints in certain brain regions; no changes to total tau |
| Morin et al. 2021 [145] | hTau mice | CHI | Either i. 5 hit rmTBI (5 injuries over 9 days with a 48-hr interval) or ii. chronic rmTBI (24 injuries, 2 per week, with a 3-4 day interval) with or without 3 month delayed anatabine treatment for 3 months | 12-14 weeks | IHC | 6 months PI | Increased pTau in both rmTBI models; anatabine decreased pTau to sham levels |
| Xu et al. 2021 [146] | WT male mice | Closed skull controlled cortical impact (CCI) | 5 impacts, 1/day for 5 consecutive days | 8-10 weeks | IHC | 1, 4, and 10 weeks PI | Increased pTau at 4 and 10 weeks PI in rmTBI mice |
| Hiskens et al. 2021 [147] | WT male mice | WD | i) a single impact (1-IMP); ii) five total impacts (5-IMP); iii) 15 total impacts (15-IMP) | - | ELISA | 2 days or 3 months PI | No differences in pTau or tau pathology |
| Kahriman et al. 2021 [148] | WT male mice | CHI | 5 hits, 1/day for 5 consecutive days | 8-12 weeks | IHC | 1, 4, or 24 weeks PI | Increased pTau-immunoreactive astrocytes and neurons and cortical pTau at 4 weeks and 24 weeks PI |
| Kahriman et al. 2022 [149] | WT male mice | WD
| 5 hits, 1/day for 5 consecutive days | 9-12 weeks | IHC | 28 days PI | Increased tau pathology in rTBI mice |
| Wu et al. 2022 [150] | WT and Interleukin 1 Receptor 1 kockout (IL1R1-/-) mice | CHI | 3 hit, 1 per consecutive day over alternating hemisphere beginning with right | 38 days | WB, IHC | ~13 months PI | Increased tau hyperphosphorylation and aggregation in male neurons but not female neurons; no differences in total tau |
| Yoon et al. 2022 [151] | WT male mice | WD | 5 hits, 1 administered every 3 days | - | IHC | 3 days PI | Increased pTau in the olfactory bulb |
| Juan et al. 2022 [152] | WT mice | CHI | Single or 5 hits administered every 48 hours | 12 weeks | WB, IHC | 1 month PI | No tau pathology differences, but tau regulatory proteins significantly altered in rmTBI mice |
| Morin et al. 2022 [153] | hTau mice | CHI | 5 consecutive mTBI over 9 days (48 h interval between the injuries) | 3 months | Mass spectrometry of isolated cortical proteins and phosphoproteins | 3 or 24 weeks PI | Increased tau associated protein in rmTBI group along with alterations to phosphoproteins; same model previously demonstrated increased tau pathology up to 2 years PI |
Table 1: Literature on CTE preclinical rodent models. Search terms for table: (chronic traumatic encephalopathy) AND model* OR (repetitive traumatic brain injury) AND tau on Pubmed.gov; refined by years 2020-2022 with focus on tau pathological assessment
Alzheimer’s disease and CTE both have strong connections with neurotrauma. Emerging data has looked at preceding inflammatory cascades that contribute to disease progression. In this review, we highlighted the novel clinical trials, innovative pre-clinical studies, and shared mechanisms between the disease states. This review offers a concise resource for clinicians and research personnel.
Declarations of Interest: None
Funding: This research did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.
Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.
Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti
Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation. “The peer review process was efficient and constructive, and the editorial office provided excellent communication and support throughout. The journal ensures scientific rigor and high editorial standards, while also offering a smooth and timely publication process. We sincerely appreciate the work of the editorial team in facilitating the dissemination of innovative approaches such as the Bonori Method.” Best regards, Dr. Matteo Bonori.

I recommend without hesitation submitting relevant papers on medical decision making to the International Journal of Clinical Case Reports and Reviews. I am very grateful to the editorial staff. Maria Emerson was a pleasure to communicate with. The time from submission to publication was an extremely short 3 weeks. The editorial staff submitted the paper to three reviewers. Two of the reviewers commented positively on the value of publishing the paper. The editorial staff quickly recognized the third reviewer’s comments as an unjust attempt to reject the paper. I revised the paper as recommended by the first two reviewers.
