AUCTORES
Globalize your Research
Research Article | DOI: https://doi.org/10.31579/2690-4861/332
Dokuz Eylül University, Engineering Faculty, Department of Environmental Engineering, Tınaztepe Campus, 35160 Buca/Izmir, Turkey.
*Corresponding Author: Delia Teresa Sponza, Dokuz Eylül University, Engineering Faculty, Department of Environmental Engineering, Tınaztepe Campus, 35160 Buca/Izmir, Turkey.
Citation: Öztekin R., Delia T. Sponza, (2023), H2 Production from Real Wastes of Polyethylene Terephthalate and Polylactic Acid using CNx/Ni2P Nanocatalyst, International Journal of Clinical Case Reports and Reviews, 14(4); DOI:10.31579/2690-4861/332
Copyright: Öztekin R., Delia T. Sponza, (2023), H2 Production from Real Wastes of Polyethylene Terephthalate and Polylactic Acid using CNx/Ni2P Nanocatalyst, International Journal of Clinical Case Reports and Reviews, 14(4); DOI:10.31579/2690-4861/332
Received: 07 August 2023 | Accepted: 24 August 2023 | Published: 01 September 2023
Keywords: carbon nitride/nickel phosphide (CNx/Ni2P) nanocatalyst; hydrogen production; microplastics; photocatalysis; polyethylene terephthalate; polylactic acid
In this study, H2(g) production from real wastes of polyethylene terephthalate and polylactic acid using CNx/Ni2P nanocatalyst was investigated with photoreforming process. Optimum experimental conditions were found at ultrasonicated 4.8 mg/ml CNx/Ni2P nanocatalyst, at pre-treated 20 mg/ml polyethylene terephthalate, at pre-treated 20 mg/ml polylactic acid, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at 60 h photoreforming solar irradiation time, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively. XRD, FESEM, EDX, FTIR, TEM, DRS and XPS analyzes were performed for characterization studies of microplastics. Polyethylene terephthalate and polylactic acid measurements were measured in inductively coupled plasma mass spectrometry (ICP-MS). H2(g) measurements were made in gas chromatography–mass spectrometry (GC-MS). The maximum 41.40 ± 5.10 and 48.60 ± 3.12 µmol H2 / gsub yields were measured for CNx=20 mg/ml and for Ni2P=20 mg/ml, respectively. The maximum 18.26 ± 1.18 and 52.41 ± 7.29 µmol H2 / gsub H2(g) production yields were found for non-sonicated CNx/Ni2P and ultra-sonicated CNx/Ni2P nanocatalyst, respectively, after 24 h photoreforming solar irradition time. The maximum 123.75 ± 11.92 and 267.41 ± 24.65 µmol H2 / gsub H2(g) production yield was measured for polyethylene terephthalate and polylactic acid, respectively, after 60 h photoreforming solar irradiation time. The maximum 6.57 ± 0.87Percentage and 2.43 ± 0.38Percentage stoichiometric H2 conversion yields were observed for polyethylene terephthalate and polylactic acid, respectively, after 60 h photoreforming solar irradition time. The maximum 96 and 57 μmol H2 / gsub H2(g) yields for polyethylene terephthalate were obtained over CNx/Ni2P and H2NCNx/Ni2P, respectively, after 60 h photoreforming solar irradiation time. The maximum 182 and 173 μmol H2 / gsub H2(g) yields for polylactic acid were observed over CNx/Ni2P and H2NCNx/Ni2P, respectively, after 60 h photoreforming solar irradiation time. The maximum 4.85 ± 0.62, 88.37 ± 10.74, 26.55 ± 1.95, 21.94 ± 1.86, 75.30 ± 9.34, 60.07 ± 5.11 and 14.61 ± 2.14 μmol H2 / gsub H2(g) production yields were obtained for Acetate, Ethylene glycol, Formate, Glycolate, Glyoxal, Lactate and Terephthalate oxidation intermediates, respectively, after 24 h photoreforming solar irradiation times. 126 nmol Acetate, 131 nmol Formate, 5 nmol Glycolate and 6200 nmol Glyoxal organic oxidation intermediates for polyethylene terephthalate with CNx/Ni2P nanocatalyst were found after 7 days photoreforming solar irradiation time. 67 nmol Acetate and 63 nmol Formate organic oxidation intermediates for polylactic acid with CNx/Ni2P nanocatalyst were obtained after 7 days photoreforming solar irradiation time. Photoreforming process is a very effective, easy to apply, economical and environmentally friendly method for the removal of plastic and microplastic wastes.
86Percentage of plastic packages are collected in landfills, or they are randomly mixed into the environment (Garcia et al., 2017; Geyer et al., 2017; MacArthur, 2017a; MacArthur, 2017b). Plastic pollution is not only a global environmental pollution problem; it also shows the unconscious waste of a very valuable resource that can be recycled and reused. The majority of polymers are synthesized from fossil fuels, especially petroleum derivatives (Garcia et al., 2017). It is estimated that approximately 3.5 billion barrels of oil can be saved each year if all global plastic waste is recycled (Garcia et al., 2017). The most important problems in the widespread application of plastic recycling are; suboptimal waste management, lack of awareness and limited size of various chemicals, complexes and polymer products (MacArthur, 2017a; MacArthur, 2017b).
Pieces of polymer smaller than ≤ 5 mm are defined as microplastics; and for recycling, microplastics represent a particularly problematic group of plastics (Cozar et al., 2014; Law and Thompson, 2014; Andrady, 2015). Microplastics formed when plastic degrades over time; They are available in a wide variety of products (Law and Thompson, 2014). Very small sizes and very dilution of microplastics; making it very difficult to collect and reuse salts from the oceans (Cozar et al., 2014; Law and Thompson, 2014; Andrady, 2015), drinking water and salts (Kosuth et al., 2018; Mason et al., 2018; Pivokonsky et al., 2018) from almost all parts of the world. A disadvantage is that even the recycling of reusable plastics has some limitations. For this reason, many polymers can only be converted into lower quality products. To give an example, only 7Percentage of recycled poly (ethylene terephthalate) (PET) bottles can be produced as re-bottles (MacArthur, 2017a). Management structures used in plastic recycling; currently far from being able to sustainably and economically treat a wide variety of plastic waste. In order to overcome these problems: in order to be able to convert the polymers at the end of use into valuable products; more functional and usable new technologies are urgently needed.
A new technology that has recently started to be used in the recovery of plastics and especially microplastics; It is a photoreformation method in which sunlight and a photocatalyst produce H2(g) from an organic substrate and water. The basic principle in the photoreformation method is that the substrate is oxidized to other organic molecules by the excited photocatalyst, acting as an electron donor. Photogenerated electrons are transferred from the photocatalyst to a co-catalyst in the next step; they reduce water to H2(g). H2(g) is in very high demand in the agricultural, pharmaceutical and chemical industries, as well as in renewable energy applications; It is a very valuable raw material (DOE, 2013; IEA, 2015). One of the existing H2(g) production technologies; In contrast to fossil fuels' steam reforming (Spath and Mann, 2001) or thermal-based approaches to converting plastic to oil (Chen et al., 2019), the key advantages of PR are; They can be run at ambient temperature and pressure, use sunlight as their sole energy input, and produce fuel cell grade H2(g). (Wakerley et al., 2017). While the photocatalytic degradation of plastics, typically to microplastics and CO2(g), has been investigated for years (Ohtani et al., 1992; Horikoshi et al., 1998; Tofa et al., 2019), not only plastic waste is reduced by the phtoreformation method; At the same time, a very important advantage is provided by producing valuable chemical products. The thermodynamics of the photoreformation process is also almost energy neutral (Kuehnel and Reisner, 2018): Photoreformation of ethylene glycol at 25oC requires ΔG° = 9.2 kJ mol-1 and E°cell = -0.01 V.
Although the photoreformation of simple molecules and biomass has been investigated in detail (Puga, 2016; Pellegrin and Odobel, 2017; Kuehnel and Reisner, 2018), plastic substrates have been largely ignored. Making polymer recycling difficult; Complex structures, low water solubility and poor biodegradation also make the photoreformation method more difficult. There are only a few previous studies on the photoreformation of plastics: in one of them; While using expensive and ultraviolet (UV) absorbing TiO2/Pt photocatalyst (Kawai and Sakata, 1981), in another; toxic CdS/CdOx quantum dots are used (Uekert et al., 2018).
Since the polymeric carbon nitride has a composition based on CNx, high stability, well-positioned conductivity (CB) and valence bands (VB) and the elements Carbon (C) and Nitrogen (N), which are abundant in the soil; It is of great interest as a new generation photocatalyst (Wang et al., 2009; Vilela et al., 2012; Cao et al., 2015; Gong et al., 2015). In recent years, CNx has been proven to support solar-powered organic substrate oxidation (Su et al., 2010), pollutant degradation (Cui et al., 2012; Qiu et al., 2015), H2(g) production (Maeda et al., 2009; Caputo et al., 2015; Lau et al., 2016), and water decomposition (Wang et al., 2009; Liu et al., 2015; Zhu et al., 2017). Photocatalytic performances; is limited to the rapid recombination of photogenerated hole-electron pairs. To overcome this limitation; Various strategies such as nonmetal doping (Zhang et al., 2010; Wang et al., 2011; Li et al., 2012), noble metal doping (Ding et al., 2011; Gao et al., 2016; Li et al., 2016) and nanoengineering of CNx (Yang et al., 2013; Zhan et al., 2017) can be applied. As an alternative strategy, it may be aimed to design a system in which photogenerated holes or electrons are consumed faster than charge recombination reactions by accelerating charge transfer to the catalytic sites.
Carbon nitride is a non-toxic and very affordable polymeric photocatalyst (Thomas et al., 2008; Wang et al., 2009; Lin et al., 2019); With the addition of cyanamide defects, the photocatalytic efficiency can be increased (Lau et al., 2016). CNx has visible light absorption and band edges suitable for photoreformation reactions; CB= - 0.5 V-NHE (normal ized hydrogen electrode), VB=2.2 V-NHE has a permissive band gap of 2.7 eV (Meyer et al., 2017). It is stated in the literature that CNx is also used for photoreformation of biomass with various cocatalysts over a wide pH range (Kasap et al., 2018). With its key advantages of visible light absorption, alkalinity stability, low cost and non-toxicity, CNx is at a level that can compete with both CdS/CdOx and TiO2/Pt for polymer photoreformation. Ni2P has previously been used for H2(g) formation with nonfunctional carbon nitride (H2NCNx) and a soluble sacrificial electron donor (triethanolamine) evolution (Indra et al., 2017; Ye et al., 2017; Wen et al., 2017), and has high potential for plastic PR given its alkaline compatibility and relatively high H2(g) forming activity (Cao et al., 2017).
Kasap et al. (2016), in order to simultaneously produce aldehyde and H2(g) from alcohol in 1/1 stoichiometry; investigated a melon type carbon nitride (NCNCNx) with cyanamide surface function in combination with a molecular nickel (II) bis(diphosphine) H2(g) evolution catalyst NiP, oxidation and proton reduction, respectively. This closed photocatalytic redox system exhibited enhanced photoactivity with NCNCNx compared to the unfunctionalized (amino-terminated) carbon nitride H2NCNx with superior hole quenching ability of 4-methyl benzyl alcohol (4-MBA). The slow transfer of photogenerated electrons to diffusional NiP has been observed as the overall rate-limiting step for this photocatalyst system (Kasap et al., 2016).
A noble metal-free sacrificial photocatalytic system using dysfunctional H2NCNx and molecular catalyst NiP was investigated (Caputo et al., 2014). When NiP is fixed in a homogeneous solution and on semiconductor surfaces (Gross et al., 2014; Leung et al., 2017; Creissen et al., 2018); It is a hydrogenase-inspired molecular H2(g)-evolution catalyst (Helm et al., 2011; Kilgore et al., 2011) with good activity. Catalyzed by a more active and surface functionalized NCNCNx and sacrificial electron donor NiP; It is modified by a selective and almost quantitative alcohol oxidation reaction due to proton reduction (Kasap et al., 2016).
In this study, production of H2(g) from polyethylene terephthalate and polylactic acid wastes using CNx/Ni2P nanocatalyst was investigated. In our experimental study, polyester microfibers and real non-recyclable plastic waste, including those contaminated with oil, were used. XRD, FESEM, EDX, FTIR, TEM, DRS and XPS analyzes were performed for characterization studies of microplastics. Polyethylene terephthalate and polylactic acid measurements were measured in inductively coupled plasma mass spectrometry (ICP-MS). H2(g) measurements were made in gas chromatography–mass spectrometry (GC-MS).
Nonfunctionalized carbon nitride (H2NCNx) was prepared by slow heating of melamine to 550°C for 3 h in a ventilated environment according to the literature procedure (Liu et al., 2015). The resulting compound was ground and powdered in a mortar. Combining cyanamide-functional CNx, H2NCNx and KSCN (weight ratio=1/2); It was prepared by heating under Ar gas for 1 h at 400°C and then at 500°C for 30 min (Lau et al., 2016). After cooling the powder was washed with deionized H2O and dried under vacuum at 60°C.
NiCl2.6H2O and NaH2PO2.H2O (weight ratio=1/5) were mixed in water for 1 h and then subjected to an ultrasonic bath for 1 h; finally dried under vacuum at 60°C. The dry compound was heated at 200°C for 1 h under Ar gas with a ramp rate of 5°C/min. After cooling to 25oC, it was washed with 2 units of black powder, 2 units of deionized H2O and 1 unit of ethanol; It was dried under vacuum at 60°C.
According to the procedure described in the literature (Indra et al., 2017), CNx, H2NCNx or TiO2 nanoparticles (NPs) (300 mg) and 20 mg NiCl2.6H2O were taken and combined in 1 ml of deionized H2O; It was stirred for 1 h and then sonicated for 1 h. Then, 100 mg of NaH2PO2.H2O was added to the mixture, mixed again and sonicated for 1 h. This mixture was dried under vacuum at 60°C and heated under Ar(g) with a rise rate of 5°C/min at 200°C for 1 h. After cooling to 25oC, the powder mixture; washed with 3 parts of deionized H2O and 3 parts of ethanol, respectively, and dried under vacuum at 60°C.
50 mg/l polymer was mixed at 300 rpm with our adaptation from the procedure described in the literature (Uekert et al., 2018). Then put in a closed bottle; It was immersed in 2 M aqueous semiconductor grade KOH at 40oC for 24 h.
A dispersion of CNx/Ni2P nanocatalyst in 5 mg/ml deionized H2O; 10 minutes were ultrasonicized with 30 second pulses at 100Percentage amplitude followed by 5 second pauses (Kasap et al., 2018). For 0.65 ml mixture in 2 M aqueous semiconductor grade KOH; 1 ml of pretreated polymer and 0.35 ml of deionized H2O per sample were used. Optimal experimental conditions were determined for 4 ml of 1 M KOH, 4.8 mg/ml CNx/Ni2P nanocatalyst, 50 mg/ml polymer, 20 mg/ml polyethylene terephthalate bottle or 15 mg/ml polyester microfibers, respectively. CNx/Pt nanocomposites (NCs); It was made by ultrasonicating CNx and then adding H2PtCl6 as a precursor, while Pt was synthesized via in situ photodeposition. Prepared samples were added to Pyrex glass photoreactor bottles with an internal volume of 10 ml and the bottles were tightly closed with rubber septa caps. After a short vortexing the samples; For 10 min GC grade analysis, it was cleaned by sparging with N2(g) containing 2Percentage CH4(g) and no CH4(g) was found in the samples after illumination without adding this internal standard. The samples were equipped with an AM 1.0 global (G) air mass filter and to remove infrared radiation; irradiated with a sunlight simulator (Newport/Oriel Sol3A Class AAA Solar Simulator, Model 94043A, 150 mW/cm2) equipped with a water filter. Visible light experiments were performed by adding a cut-off filter for λ Greater-than 400 nm. All samples were mixed at 600 rpm and held constant at 25°C during irradiation. Considering 40 µl of reactor headspace gas for each sample; H2(g) production in the samples was analyzed periodically by GC-MS. Excessive pressure inside the bottle; minimally observed, with an increase of 0.02 atm per 10 μmol H2(g) produced.
4 ml of 1 M KOH is taken; Samples containing 10 mg of substrate were prepared and irradiated for photocatalysis. H2(g) conversion (Percentage) were calculated as in Equation 1 (Emel’yanenko et al., 2010; NIST Chemistry WebBook, 2023):
 (1)
(1)
where; nH2, exp: is the H2(g) measured in experiment (mol), n substrate, exp: is the substrate used in experiment (mol), and nH2, idealn substrate, ideal −1: is the ideal ratio of moles H2(g) to substrate, respectively.
Power output from generated H2(g); It was calculated according to Equation 2:
 (2)
(2)
where; VH2: is the molar volume of H2(g) (24.47 l/mol at 25oC), nH2: is the moles of H2(g) produced, ρH2: is the density of H2(g) (8.235×10−5 kg/l at 25oC), uH2: is the lower heating value of H2(g) (120×106 J/kg), and tirr: is the irradiation time (s), respectively.
2.8. Characterization
2.8.1. X-Ray Diffraction (XRD) Analysis
Powder XRD patterns were recorded on a Shimadzu XRD-7000, Japan diffractometer using Cu Kα radiation (λ = 1.5418 Å, 40 kV, 40 mA) at a scanning speed of 1o /min in the 10-80o 2θ range. Raman spectrum was collected with a Horiba Jobin Yvon-Labram HR UV-Visible NIR (200-1600 nm) Raman microscope spectrometer, using a laser with the wavelength of 512 nm. The spectrum was collected from 10 scans at a resolution of 2 /cm. The zeta potential was measured with a SurPASS Electrokinetic Analyzer (Austria) with a clamping cell at 300 mbar.
2.8.2. Field Emission Scanning Electron Microscopy (FESEM)
The morphological features and structure of the experimental samples were determined by Field Emission Scanning Electron Microscopy (FESEM) (FESEM, Hitachi S-4700).
2.8.3. Energy Dispersive X-Ray (EDX) Spectroscopy Analysis
The elements on the surface of the experimental samples were analyzed using energy dispersive X-ray analysis (EDX) with EDX spectrometry device (TESCAN Co., Model III MIRA).
2.8.4. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
The FTIR spectra of experimental samples were recorded using the FT-NIR spectroscope (RAYLEIGH, WQF-510).
2.8.5. Transmission Electron Microscopy (TEM) Analysis
The obtained experimental samples were collected and harvested by centrifugation (8000 rpm, 5 min), washed twice with deionized H2O, and resuspended in ethanol (C2H6O) and dripped onto a carbon-coated copper (Cu) Transmission Electron Microscopy (TEM) grid. Vacuum drying then occurred to the experimental samples for 24 h at 25oC room temperature. The dry samples on the Cu grid were viewed and examined by TEM Analysis recorded in a JEOL JEM 2100F, Japan under 200 kV accelerating voltage. The size and structure of the experimental samples were identified with TEM analysis.
2.8.6. Diffuse Reflectance UV-Vis Spectra (DRS) Analysis
DRS Analysis in the range of 200–800 nm was recorded on a Cary 5000 UV-Vis Spectrophotometer from Varian. DRS was used to monitor the experimental samples.
2.8.7. X-Ray Photoelectron Spectroscopy (XPS) Analysis
The valence state of the experimental samples were investigated and was analyzed using XPS (ESCALAB 250Xi, England). XPS used an Al Ka source and surface chemical composition and reduction state analyses was done, with the core levels recorded using a pass energy of 30 eV (resolution approximately 0.10 eV). The peak fitting of the individual core-levels was done using XPS-peak 41 software, achieving better fitting and component identification. All binding energies were calibrated to the C 1s peak originating from C–H or C–C groups at 284.6 eV
2.8.8. Inductively Coupled Plasma Mass Spectrometry (ICP-MS) Analysis
The Agilent 8800 ICP-MS instrument (Agilent Technologies, Japan) was used to determine different concentrations of polyethylene terephthalate and polylactic acid. For analysis, the samples were acid digested in closed Savillex® PFA beakers prior to ICP-MS. 5 ml of suspended sample in PBS was centrifuged at 10000 ×g for 30 min, after which the supernatant was removed. The resulting pellet was acid digested with 1.5 ml of 14 mol/l HNO3 and 0.5 ml of 9.8 mol/l H2O2. The closed beakers were heated to 115°C overnight on a hot plate. After complete mineralization, the digestates were evaporated at 90°C until dryness, then redissolved in 2.0 ml of 0.35 mol/l HNO3. This solution was further diluted with 0.35 mol/l HNO3 and rhodium (Rh) was added as the internal standard (final concentration = 2 μg/l) to compensate for potential matrix effects and/or signal instability.
2.8.9. Gas Chromatography–Mass Spectrometry (GC-MS)
Gas chromatography–mass spectrometry (GC-MS) and gas chromatograph (GC) (Agilent Technology model 6890N) equipped with a mass selective detector (Agilent 5973 inert MSD). Mass spectra were recorded using a VGTS 250 spectrometer equipped with a capillary SE 52 column (HP5-MS 30 m, 0.25 mm ID, 0.25 μm) at 220°C with an isothermal program for 10 min. The initial oven temperature was kept at 50oC for 1 min, then raised to 220oC at 25oC/min and from 200 to 300oC at 8oC/min, and was then maintained for 5.5 min. High purity He (g) was used as the carrier gas at constant flow mode (1.5 ml/min, 45 cm/s linear velocity). All H2(g) measurements of the experimental samples were made in the GC-MS device.
The results of XRD analysis were observed after H2(g) production from real wastes of polyethylene terephthalate and polylactic acid using CNx/Ni2P nanocatalyst with photoreforming process (Figure 1). The characterization peaks were found at 2θ values of 7.74o, 46.10o, 28.16o, 36.11o and 43.87o, respectively, and which can also be indexed as (101), (210), (204), (312) and (222), respectively (Figure 1).
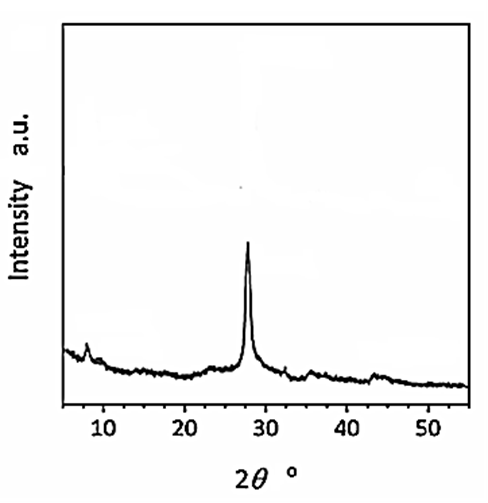
Figure 1: XRD spectra of CNx/Ni2P nanocatalyst after photoreforming process for H2(g) production from real wastes of polyethylene terephthalate and polylactic acid.
The morphological features of CNx/Ni2P nanocatalyst was characterized through FESEM images before photoreforming process (Figure 2a) and after photoreforming process (Figure 2b), respectively.
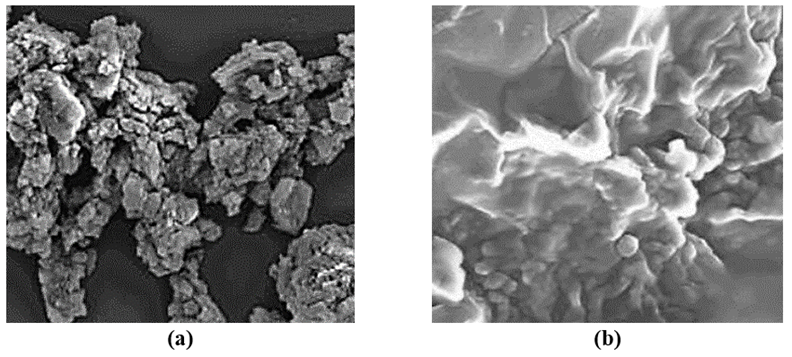
Figure 2: FESEM images of CNx/Ni2P nanocatalyst (a) before photoreforming process (b) after photoreforming process for H2(g) production from real wastes of polyethylene terephthalate and polylactic acid (FESEM size: 4 μm).
The results of EDX analysis were found after H2(g) production from real wastes of polyethylene terephthalate and polylactic acid using CNx/Ni2P nanocatalyst with photoreforming process (Figure 3).

Figure 3: EDX spectrum of CNx/Ni2P nanocatalyst after photoreforming process for H2(g) production from real wastes of polyethylene terephthalate and polylactic acid.
The FTIR spectrum of CNx/Ni2P nanocatalyst was measured to H2(g) production from real wastes of polyethylene terephthalate and polylactic acid after photoreforming process (Figure 4). The main peaks of FTIR spectrum for CNx/Ni2P nanocatalyst was observed at 3700 1/cm, 2250 1/cm, 2010 1/cm, 1870 1/cm, 1600 1/cm, 1150 1/cm, 960 1/cm and 830 1/cm wavenumber, respectively (Figure 4).
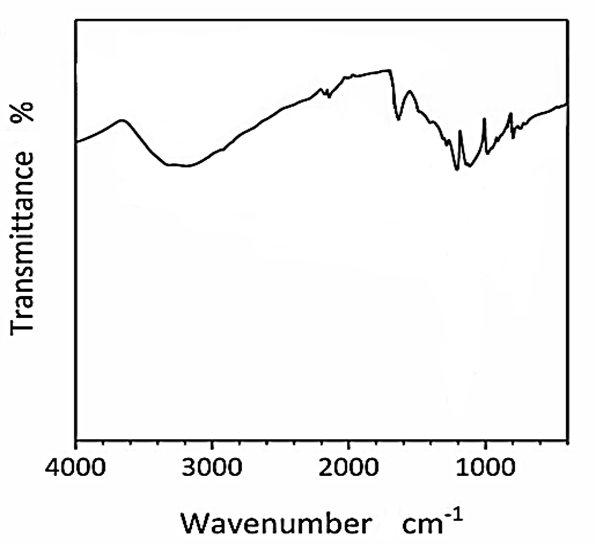
Figure 4: FTIR spectrum of CNx/Ni2P nanocatalyst after photoreforming process for H2(g) production from real wastes of polyethylene terephthalate and polylactic acid.
The TEM images of CNx/Ni2P nanocatalyst was obtained to H2(g) production from real wastes of polyethylene terephthalate and polylactic acid after photoreforming process (Figure 5).

Figure 5: TEM images of CNx/Ni2P nanocatalyst after photoreforming process for H2(g) production from real wastes of polyethylene terephthalate and polylactic acid.
The DRS spectrum of CNx/Ni2P nanocatalyst was observed to H2(g) production from real wastes of polyethylene terephthalate and polylactic acid after photoreforming process (Figure 6). The DRS spectra of CNx/Ni2P nanocatalyst was recorded in the wavelength range from 300 nm to 600 nm using diffuse reflectance UV-V is spectra (Figure 6). The DRS spectrum absorption peaks of CNx/Ni2P nanocatalyst were found at wavelengths of 310 nm, 320 nm, 335 nm, 348 nm and 425 nm, respectively, for CNx/Ni2P nanocatalyst after photoreforming process (Figure 6).
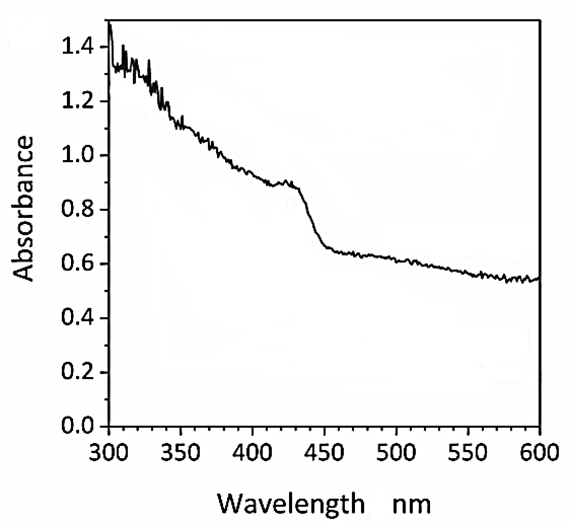
Figure 6: The DRS spectrum of CNx/Ni2P nanocatalyst after photoreforming process for H2(g) production from real wastes of polyethylene terephthalate and polylactic acid.
The XPS analysis of CNx/Ni2P nanocatalyst was found to H2(g) production from real wastes of polyethylene terephthalate and polylactic acid after photoreforming process (Figure 7). The results of XPS analysis were measured for N1s edge of CNx/Ni2P nanocatalyst (Figure 7a) and P2p edge of CNx/Ni2P nanocatalyst (Figure 7b), respectively. The binding energy value for N1s edge of CNx/Ni2P nanocatalyst was measured to 398.76 eV for N=C bond and 400.95 eV for N-C bond, respectively (Figure 7a). The binding energy value for P2p edge of CNx/Ni2P nanocatalyst was observed to 133.11 eV for POx (Figure 7b).

(a)
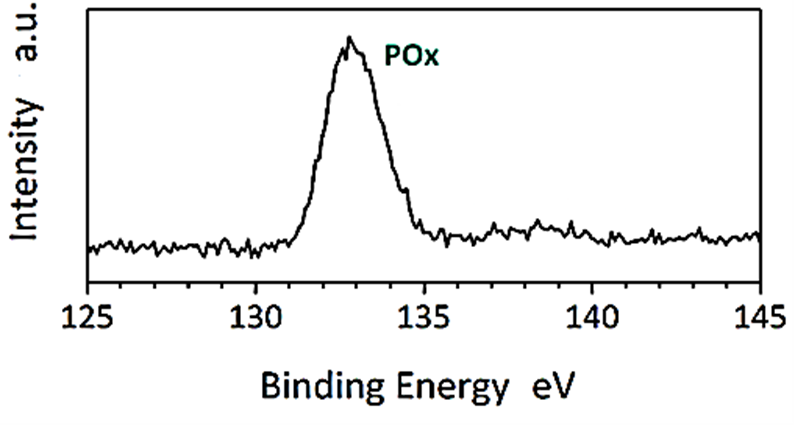
(b)
Figure 7: XPS spectra of (a) N1s edge of CNx/Ni2P nanocatalyst and (b) P2p edge of CNx/Ni2P nanocatalyst after photoreforming process for H2(g) production from real wastes of polyethylene terephthalate and polylactic acid.
Photoreforming conditions for H2(g) production from real wastes of polyethylene terephthalate and polylactic acid with CNxNi2P nanocatalyst was summarized at Table 1. The maximum 41.40 ± 5.10 µmol H2 / gsub yield was measured for CNx=20 mg/ml, at ultrasonicated 4.8 mg/ml CNx/Ni2P nanocatalyst, at pre-treated 20 mg/ml polyethylene terephthalate, at pre-treated 20 mg/ml polylactic acid, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at 24 h, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively (Table 1).
| Parameters (Unit) | Concentrations | Activity (µmol H2 / gcat .h) | H2(g) Yield (µmol H2 / gsub) |
| CNx (mg/ml)) | 5 | 2.39 ± 0.15 | 1.47 ± 0.45 |
| 10 | 32.40 ± 4.05 | 2.18 ± 0.375 | |
| 20 | 34.65 ± 4.82 | 41.40 ± 5.10 | |
| 50 | 33.74 ± 4.52 | 33.15 ± 1.65 | |
| Ni2P (mg/ml) | 5 | 9.18 ± 2.81 | 3.17 ± 0.19 |
| 10 | 13.68 ± 0.70 | 41.40 ± 5.10 | |
| 20 | 35.17 ± 5.4 | 48.60 ± 3.12 | |
| 50 | 30.17 ± 3.73 | 44.10 ± 2.25 | |
| Note: Yields and activities are cumulative values. The standard deviation (σ) calculated from 3 samples. | |||
Table 1: Photoreforming conditions for H2(g) production from real wastes of polyethylene terephthalate and polylactic acid with CNxNi2P nanocatalyst (Experimental conditions: at ultrasonicated 4.8 mg/ml CNx/Ni2P nanocatalyst, at pre-treated 20 mg/ml polyethylene terephthalate, at pre-treated 20 mg/ml polylactic acid, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at 24 h, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively).The maximum 48.60 ± 3.12 µmol H2 / gsub yield was measured for Ni2P=20 mg/ml, at ultrasonicated 4.8 mg/ml CNx/Ni2P nanocatalyst, at pre-treated 20 mg/ml polyethylene terephthalate, at pre-treated 20 mg/ml polylactic acid, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at 24 h, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively (Table 1).
Optimization was achieved for maximum total H2(g) production under all experimental conditions (CNx loading, Ni2P loading, CNx/Ni2P photocatalyst concentration, polyethylene terephthalate, polylactic acid, polymer concentration, photoreforming time, temperature, pH, and irradiation solar light conditions…etc.) (Table 1). Harsh conditions (e.g., high pH) are often required to solubilize plastic, and polymer photoreforming withCNx/Ni2P improves significantly with increasing pH values, from 41.40 μmol H2 / gsub, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at 24 h, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively (Table 1).
Comparison of photoreforming with non-sonicated versus ultra-sonicated CNx/Ni2P nanocatalyst was determined at Table 2. Experimental conditions were optimized at 4.8 mg/ml CNx/Ni2P, 20 mg/ml polyethylene terephthalate, pre-treated 20 mg/ml polylactic acid, 4 ml aqueous 1 M KOH, 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at 24 h, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively (Table 2).
The CNx/Ni2P nanocatalyst was ultrasonicated in H2O for 10 min according to a procedure reported in the literature (Kasap et al., 2018). This ultrasonication process is known to increase the surface area and activity of the CNx/Ni2P nanocatalyst (Kasap et al., 2018).
| Parameters | Time (h) | Activity (µmol H2 / gcat .h) | H2(g) Yield (µmol H2 / gsub) |
| None sonication | 6 | 22.65 ± 1.20 | 11.59 ± 0.57 |
| 12 | 16.88 ± 0.77 | 13.42 ± 0.88 | |
| 18 | 7.43 ± 0.53 | 15.28 ± 1.09 | |
| 24 | 4.60 ± 0.37 | 18.26 ± 1.18 | |
| With sonication | 6 | 29.71 ± 1.96 | 22.06 ± 1.09 |
| 12 | 31.44 ± 3.78 | 24.89 ± 1.56 | |
| 18 | 33.57 ± 4.12 | 41.54 ± 5.11 | |
| 24 | 37.68 ± 4.94 | 52..41 ± 7.29 | |
| Note: Yields and activities are cumulative values. The standard deviation (σ) calculated from 3 samples. | |||
Table 2: Comparison of photoreforming with non-sonicated versus ultra-sonicated CNx/Ni2P nanocatalyst (Experimental conditions: at 4.8 mg/ml CNx/Ni2P, at pre-treated 20 mg/ml polyethylene terephthalate, at pre-treated 20 mg/ml polylactic acid, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at 24 h, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively).
The maximum 18.26 ± 1.18 µmol H2 / gsub H2(g) production yield of non-sonicated CNx/Ni2P nanocatalyst was observed during photoreforming process, after 24 h photoreforming solar irradition time, at 4.8 mg/ml CNx/Ni2P, at 20 mg/ml polyethylene terephthalate, at pre-treated 20 mg/ml polylactic acid, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively (Table 2) The maximum 52.41 ± 7.29 µmol H2/gsub H2(g) production yield of ultra-sonicated CNx/Ni2P nanocatalyst was measured during photoreforming process, after 24 h photoreforming solar irradition time, at 4.8 mg/ml CNx/Ni2P, at 20 mg/ml polyethylene terephthalate, at pre-treated 20 mg/ml polylactic acid, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively (Table 2).
The photoreforming of pre-treated polyethylene terephthalate and pre-treated polylactic acid with CNx/Ni2P was obtained at Table 3.Photoreforming experimental conditions was optimized at ultra-sonicated 4.8 mg/ml CNx/Ni2P, at pre-treated 50 mg/ml polymer, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at 24 h, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively.
| Parameters | Time (h) | Activity (µmol H2 / gcat .h) | H2(g) Yield (µmol H2 / gsub) |
Polyethylene Terephthalate | 6 | 35.75 ± 1.25 | 5.86 ± 0.29 |
| 12 | 38.27 ± 1.95 | 9.80 ± 0.50 | |
| 18 | 39.70 ± 2.03 | 49.66 ± 4.55 | |
| 24 | 41.45 ± 3.15 | 63.16 ± 7.04 | |
| 36 | 44.32 ± 3.94 | 68.14 ± 7.39 | |
| 48 | 51.36 ± 5.02 | 72.00 ± 6.90 | |
| 54 | 55.24 ± 5.82 | 108.5 ± 11.25 | |
| 60 | 60.77 ± 6.39 | 123.75 ± 11.92 | |
Polylactic Acid | 6 | 64.50 ± 7.15 | 8.25 ± 0.41 |
| 12 | 68.05 ± 7.32 | 14.90 ± 1.05 | |
| 18 | 69.96 ± 7.45 | 89.55 ± 9.06 | |
| 24 | 72.90 ± 7.78 | 116.74 ± 11.48 | |
| 36 | 75.04 ± 8.05 | 129.64 ± 12.36 | |
| 48 | 83.11 ± 9.30 | 234.15 ± 18.54 | |
| 54 | 84.70 ± 9.65 | 246.32 ± 22.82 | |
| 60 | 87.53 ± 9.92 | 267.41 ± 24.65 | |
| Note: Yields and activities are cumulative values. The standard deviation (σ) calculated from 3 samples. | |||
Table 3: Photoreforming of pre-treated polyethylene terephthalate and pre-treated polylactic acid with CNx/Ni2P (Experimental conditions: at ultra-sonicated 4.8 mg/ml CNx/Ni2P, at pre-treated 50 mg/ml polymer, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at 24 h, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively).
The maximum 123.75 ± 11.92 µmol H2 / gsub H2(g) production yield was measured during photoreforming process for polyethylene terephthalate after 60 h photoreforming solar irradiation time, at ultra-sonicated 4.8 mg/ml CNx/Ni2P, at pre-treated 50 mg/ml polymer, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively (Table 3).
The maximum 267.41 ± 24.65 µmol H2 / gsub H2(g) production yield was recorded during photoreforming process for polylactic acid after 60 h photoreforming solar irradiation time, at ultra-sonicated 4.8 mg/ml CNx/Ni2P, at pre-treated 50 mg/ml polymer, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively (Table 3). The stoichiometric H2 conversion calculations for polyethylene terephthalate and polylactic acid were calculated at Table 4. The maximum 6.57 ± 0.87Percentage stoichiometric H2 conversion yield was measured for polyethylene terephthalate during photoreforming process after 60 h photoreforming solar irradition time, at 4.8 mg/ml CNx/Ni2P, at 20 mg/ml polyethylene terephthalate, at 50 mg/ml polymer, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively (Table 4).
| Parameters | N100Percentage (mol H2 / molsub) | Time (h) | Nyield (mol H2 / molsub) | Conversion (Percentage) |
| Polyethylene Terephthalate | 15 | 0.041 ± 0.004 | 0.81 ± 0.09 | |
| 5.0 a | 30 | 0.060 ± 0.006 | 1.21 ± 0.12 | |
| 45 | 0.182 ± 0.023 | 3.63 ± 0.45 | ||
| 60 | 0.330 ± 0.044 | 6.57 ± 0.87 | ||
| Polylactic Acid | 5.0 | 15 | 0.024 ± 0.003 | 0.41 ± 0.05 |
| 30 | 0.039 ± 0.006 | 0.43 ± 0.06 | ||
| 45 | 0.092 ± 0.018 | 1.53 ± 0.30 | ||
| 60 | 0.146 ± 0.023 | 2.43 ± 0.38 | ||
| a: assumed that only the ethylene glycol component of polyethylene terephthalate is oxidized. | ||||
| Note: Yields and activities are cumulative values. The standard deviation (σ) calculated from 3 samples. | ||||
Table 4: Stoichiometric H2 conversion calculations (Experimental conditions: 4.8 mg/ml CNx/Ni2P, at pre-treated 20 mg/ml polyethylene terephthalate, at pre-treated 20 mg/ml polylactic acid, at 50 mg/ml polymer, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at 24 h, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively).
The maximum 2.43 ± 0.38Percentage stoichiometric H2 conversion yield was observed for polylactic acid during photoreforming process after 60 h photoreforming solar irradition time, at 4.8 mg/ml CNx/Ni2P, at 20 mg/ml polylactic acid, at 50 mg/ml polymer, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively (Table 4). Values reported for CdS/CdOx under the same conditions were 16.6 ± 1.0Percentage for polyethylene terephthalate and 38.8 ± 4.0Percentage for polylactic acid during photoreforming process (Uekert et al., 2018). It should also be noted that, consistent with previous reports, these calculations assume that only the aliphatic portion of the polyethylene terephthalate is oxidized during photoreforming process (Uekert et al., 2018). Comparison of photoreforming of polyethylene terephthalate and polylactic acid over CNx/Ni2P and H2NCNx/Ni2P were shown at Figure 8. 11, 19, 33, 55, 67 and 79 μmol H2 / gsub H2(g) yields for polyethylene terephthalate over CNx/Ni2P were observed after 5, 10, 20, 30, 40 and 50 h photoreforming solar irradiation times, respectively, at 4.8 mg/ml CNx/Ni2P photocatalyst, at pre-treated 20 mg/ml polyethylene terephthalate, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively (Figure 8a). The maximum 96 μmol H2 / gsub H2(g) yields for polyethylene terephthalate over CNx/Ni2P were obtained after 60 h photoreforming solar irradiation time, at 4.8 mg/ml CNx/Ni2P photocatalyst, at pre-treated 20 mg/ml polyethylene terephthalate, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively (Figure 8a). 5, 21, 38, 42, 44 and 49 μmol H2 / gsub H2(g) yields for polyethylene terephthalate over H2NCNx/Ni2P were measured after 5, 10, 20, 30, 40 and 50 h photoreforming solar irradiation times, respectively, at 4.8 mg/ml CNx/Ni2P photocatalyst, at pre-treated 20 mg/ml polyethylene terephthalate, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively (Figure 8a). The maximum 57 μmol H2 / gsub H2(g) yields for polyethylene terephthalate over H2NCNx/Ni2P were obtained after 60 h photoreforming solar irradiation time, at 4.8 mg/ml CNx/Ni2P photocatalyst, at pre-treated 20 mg/ml polyethylene terephthalate, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively (Figure 8a).
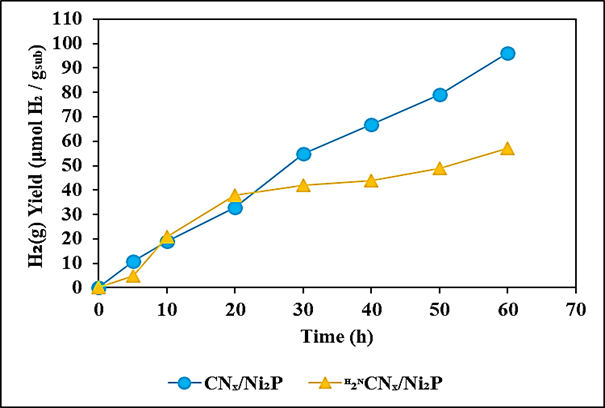
(a)

(b)
Figure 8: Comparison of photoreforming of (a) polyethylene terephthalate and (b) polylactic acid over CNx/Ni2P and H2NCNx/Ni2P. Experimental conditions: at 4.8 mg/ml CNx/Ni2P photocatalyst, at pre-treated 20 mg/ml polyethylene terephthalate, at pre-treated 20 mg/ml polylactic acid, at 4 ml aqueous 1M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at 60 h, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively.
15, 32, 66, 110, 143 and 165 μmol H2 / gsub H2(g) yields for polylactic acid over CNx/Ni2P were observed after 5, 10, 20, 30, 40 and 50 h photoreforming solar irradiation times, respectively, at 4.8 mg/ml CNx/Ni2P photocatalyst, at pre-treated 20 mg/ml polylactic acid, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively (Figure 8b). The maximum 182 μmol H2 / gsub H2(g) yields for polylactic acid over CNx/Ni2P were obtained after 60 h photoreforming solar irradiation time, at 4.8 mg/ml CNx/Ni2P photocatalyst, at pre-treated 20 mg/ml polylactic acid, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively (Figure 8b). 27, 51, 100, 118, 136 and 147 μmol H2 / gsub H2(g) yields for polylactic acid over H2NCNx/Ni2P were measured after 5, 10, 20, 30,40 and 50 h photoreforming solar irradiation times, respectively, at 4.8 mg/ml CNx/Ni2P photocatalyst, at pre-treated 20 mg/ml polylactic acid, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively (Figure 8b). The maximum 173 μmol H2 / gsub H2(g) yields for polylactic acid over H2NCNx/Ni2P were obtained after 60 h photoreforming solar irradiation time, at 4.8 mg/ml CNx/Ni2P photocatalyst, at pre-treated 20 mg/ml polylactic acid, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively (Figure 8b).
3. The By-Products Measurements of Polyethylene Terephthalate and Polylactic Acid
The GC-MS results was showed that both polyethylene terephthalate and polylactic acid form a variety of oxidation products after 12 h and 24 h photoreforming solar irradiation times, at 4.8 mg/ml CNx/Ni2P, at 20 mg/ml polyethylene terephthalate, at pre-treated 20 mg/ml polylactic acid, at 50 mg/ml polymer, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at 24 h at AM 1.0G, at 150 mW/cm2, at 25°C, respectively (Table 5).
| Intermediates | Time (h) | Activity (µmol H2 / gcat .h) | H2(g) Yield (µmol H2 / gsub) |
| Acetate | 12 | 6.56 ± 0.84 | 1.68 ± 0.21 |
| 24 | 3.77 ± 0.47 | 4.85 ± 0.62 | |
| Ethylene glycol | 12 | 75.76 ± 3.75 | 19.35 ± 1.93 |
| 24 | 69.15 ± 8.40 | 88.37 ± 10.74 | |
| Formate | 12 | 25.20 ± 1.52 | 6.45 ± 0.70 |
| 24 | 20.70 ± 1.16 | 26.55 ± 1.95 | |
| Glycolate | 12 | 21.15 ± 1.32 | 5.37 ± 0.95 |
| 24 | 17.42 ± 1.67 | 21.94 ± 1.86 | |
| Glyoxal | 12 | 61.95 ± 3.15 | 15.90 ± 0.75 |
| 24 | 58.82 ± 7.35 | 75.30 ± 9.34 | |
| Lactate | 12 | 36.31± 4.60 | 9.30 ± 1.56 |
| 24 | 47.43 ± 4.05 | 60.07 ± 5.11 | |
| Terephthalate | 12 | 1.76 ± 0.15 | 3.58 ± 0.85 |
| 24 | 6.20 ± 0.77 | 14.61 ± 2.14 | |
| Note: Yields and activities are cumulative values. The standard deviation (σ) calculated from 3 samples. | |||
Table 5: H2(g) production during photoreforming of oxidation intermediates with CNx/Ni2P from GC-MS measurements results (Experimental conditions: at 4.8 mg/ml CNx/Ni2P, at pre-treated 20 mg/ml polyethylene terephthalate, at pre-treated 20 mg/ml polylactic acid, at 50 mg/ml polymer, at 4 ml aqueous 1M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at 24 h at AM 1.0G, at 150 mW/cm2, at 25°C, respectively).
The maximum 4.85 ± 0.62, 88.37 ± 10.74, 26.55 ± 1.95, 21.94 ± 1.86, 75.30 ± 9.34, 60.07 ± 5.11 and 14.61 ± 2.14 μmol H2 / gsub H2(g) production yields were obtained for Acetate, Ethylene glycol, Formate, Glycolate, Glyoxal, Lactate and Terephthalate oxidation intermediates, respectively, after 24 h photoreforming solar irradiation times, at 4.8 mg/ml CNx/Ni2P, at 20 mg/ml polyethylene terephthalate, at pre-treated 20 mg/ml polylactic acid, at 50 mg/ml polymer, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at 24 h at AM 1.0G, at 150 mW/cm2, at 25°C, respectively (Table 5). The photoreforming process of lactic acid (the monomer of polylactic acid) is also faster on CNx/Ni2P (47.43 ± 4.05 μmol H2 /gsub. h, after 24 h photoreforming solar irradiation time at Table 5) than a reported H2NCNx/WS2 system in H2O (0.50 μmol H2 /gsub. h) (Hou et al., 2014). Quantification of organic oxidation intermediates for polyethylene terephthalate and polylactic acid with CNx/Ni2P after 7 days photoreforming process from ICP-MS measurements results were recorded at Table 6. 126 nmol Acetate, 131 nmol Formate, 5 nmol Glycolate and 6200 nmol Glyoxal organic oxidation intermediates for polyethylene terephthalate with CNx/Ni2P nanocatalyst were found after 7 days photoreforming solar irradiation time, at 4.8 mg/ml CNx/Ni2P, at 20 mg/ml polyethylene terephthalate, at 50 mg/ml polymer, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively (Table 6).
| Parameters | Organic oxidation intermediates | Quantity (nmol) |
| Polyethylene terephthalate | Acetate | 126 |
| Formate | 131 | |
| Glycolate | 5 | |
| Glyoxal | 6200 | |
| Polylactic acid | Acetate | 67 |
| Formate | 63 | |
Note 1: Yields and activities are cumulative values. The standard deviation (σ) calculated from 3 samples. Note 2: Reference standard: Maleic acid | ||
Table 6: Quantification of organic oxidation intermediates for polyethylene terephthalate and polylactic acid with CNx/Ni2P nanocatalyst after 7 days photoreforming process from ICP-MS measurements results (Experimental conditions: at 4.8 mg/ml CNx/Ni2P, at 20 mg/ml polyethylene terephthalate, at 50 mg/ml polymer, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively).
67 nmol Acetate and 63 nmol Formate organic oxidation intermediates for polylactic acid with CNx/Ni2P nanocatalyst were obtained after 7 days photoreforming solar irradiation time, at 4.8 mg/ml CNx/Ni2P, at pre-treated 20 mg/ml polylactic acid, at 50 mg/ml polymer, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively (Table 6).
The maximum 41.40 ± 5.10 and 48.60 ± 3.12 µmol H2 / gsub yields were measured for CNx=20 mg/ml and for Ni2P=20 mg/ml, respectively, with photoreforming process, at ultrasonicated 4.8 mg/ml CNx/Ni2P nanocatalyst, at pre-treated 20 mg/ml polyethylene terephthalate, at pre-treated 20 mg/ml polylactic acid, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at 24 h, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively.
The maximum 18.26 ± 1.18 and 52..41 ± 7.29 µmol H2 / gsub H2(g) production yields were found for non-sonicated CNx/Ni2P and ultra-sonicated CNx/Ni2P nanocatalyst, respectively, with photoreforming process, after 24 h photoreforming solar irradition time, at 4.8 mg/ml CNx/Ni2P, at 20 mg/ml polyethylene terephthalate, at pre-treated 20 mg/ml polylactic acid, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively. The maximum 123.75 ± 11.92 and 267.41 ± 24.65 µmol H2 / gsub H2(g) production yield was measured for polyethylene terephthalate and polylactic acid, respectively, with photoreforming process, after 60 h photoreforming solar irradiation time, at ultra-sonicated 4.8 mg/ml CNx/Ni2P, at pre-treated 50 mg/ml polymer, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively. The maximum 6.57 ± 0.87Percentage and 2.43 ± 0.38Percentage stoichiometric H2 conversion yields were observed for polyethylene terephthalate and polylactic acid, respectively, with photoreforming process, after 60 h photoreforming solar irradition time, at 4.8 mg/ml CNx/Ni2P, at 20 mg/ml polyethylene terephthalate, at 20 mg/ml polylactic acid, at 50 mg/ml polymer, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively. The maximum 96 and 57 μmol H2 / gsub H2(g) yields for polyethylene terephthalate were obtained over CNx/Ni2P and H2NCNx/Ni2P, respectively, with photoreforming process, after 60 h photoreforming solar irradiation time, at 4.8 mg/ml CNx/Ni2P photocatalyst, at pre-treated 20 mg/ml polyethylene terephthalate, at 4 ml aqueous 1 M KOH, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively. The maximum 182 and 173 μmol H2 / gsub H2(g) yields for polylactic acid were observed over CNx/Ni2P and H2NCNx/Ni2P, respectively, with photoreforming process, after 60 h photoreforming solar irradiation time, at 4.8 mg/ml CNx/Ni2P photocatalyst, at pre-treated 20 mg/ml polylactic acid, at 4 ml aqueous 1 M KOH, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively. The maximum 4.85 ± 0.62, 88.37 ± 10.74, 26.55 ± 1.95, 21.94 ± 1.86, 75.30 ± 9.34, 60.07 ± 5.11 and 14.61 ± 2.14 μmol H2 / gsub H2(g) production yields were obtained for Acetate, Ethylene glycol, Formate, Glycolate, Glyoxal, Lactate and Terephthalate oxidation intermediates, respectively, with photoreforming process, after 24 h photoreforming solar irradiation times, at 4.8 mg/ml CNx/Ni2P, at 20 mg/ml polyethylene terephthalate, at pre-treated 20 mg/ml polylactic acid, at 50 mg/ml polymer, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively. 126 nmol Acetate, 131 nmol Formate, 5 nmol Glycolate and 6200 nmol Glyoxal organic oxidation intermediates for polyethylene terephthalate with CNx/Ni2P nanocatalyst were found with photoreforming process, after 7 days photoreforming solar irradiation time, at 4.8 mg/ml CNx/Ni2P, at 20 mg/ml polyethylene terephthalate, at 50 mg/ml polymer, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively. 67 nmol Acetate and 63 nmol Formate organic oxidation intermediates for polylactic acid with CNx/Ni2P nanocatalyst were observed with photoreforming process, after 7 days photoreforming solar irradiation time, at 4.8 mg/ml CNx/Ni2P, at pre-treated 20 mg/ml polylactic acid, at 50 mg/ml polymer, at 4 ml aqueous 1 M KOH, at 10 ml internal volume of sealed photoreactor under anaerobic conditions, at 1200 W Xe solar lamp, at AM 1.0G, at 150 mW/cm2, at 25°C, respectively. High H2(g) production efficiency was obtained by photoreforming process for the production of H2(g) from real polyethylene terephthalate and polylactic acid wastes using CNx/Ni2P nanocatalyst. Photoreforming process is a very effective, easy to apply, economical and environmentally friendly method for the removal of plastic and microplastic wastes.
This research study was undertaken in the Environmental Microbiology Laboratories at Dokuz Eylül University Engineering Faculty Environmental Engineering Department, Izmir, Turkey. The authors would like to thank this body for providing financial support.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.
