AUCTORES
Globalize your Research
Review Article | DOI: https://doi.org/10.31579/2637-8914/300
Department of Agriculture/Agricultural Regulation, School of Agriculture, Fisheries and Human Science, University of Arkansas at Pine Bluff. 1200 North University Drive, Mail Slot 4913, Pine Bluff, AR 71601, USA.
*Corresponding Author: Shahidul Islam, Ph.D. Professor Director, USDA Regulatory Science Center University of Arkansas 1200 North University Drive, Mail Slot 4913 Pine Bluff, AR 71601, USA
Citation: Md Sahidur Rahman, Muhammad Abul Kalam Azad, Henry W. English, and Shahidul Islam, (2025), Exploring the Biotechnological Future of Genetically Modified (GM) Crops in U.S. Agriculture: Regulatory Challenges, Scientific Foundations, and Pathways Forward, J. Nutrition and Food Processing, 8(5); DOI:10.31579/2637-8914/300
Copyright: © 2025, Shahidul Islam. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 10 February 2025 | Accepted: 20 March 2025 | Published: 09 April 2025
Keywords: biotechnology; gmos; regulatory process; safety; public health; environmental effects
The development of genetically modified (GM) crops have revolutionized U.S. agriculture by enhancing crop productivity and sustainability. However, there is a strong global consensus on the safety of GM crops-evidenced by over 4,400 risk assessments confirming no significant difference in risk between GM and non-GM crops, yet there are substantial societal, scientific, and governmental obstacles to the future expansion and integration of GM crops. This review looks at the current state of GM crop biotechnology in the U.S., focusing on the legal frameworks that control its development, the scientific discoveries that spur innovation, and the critical challenges that need addressing to ensure continued progress. The article outlines the foundational science behind GM technologies, including gene editing, precision breeding and transgenic approaches, which have made it possible to produce crop types that are more resilient and sustainable. Furthermore, the review covers the changing regulatory environment, with special attention to the difficulties in navigating federal regulations, public perception challenges, ethical considerations, and worries about the effects on the environment and human health. Lastly, the article proposes potential pathways forward, emphasizing the necessity of regulatory change, public and policymaker education, and research funding to realize the full potential of GM crops. By addressing these challenges, U.S. agriculture can continue to benefit from biotechnological advancements while maintaining safety, sustainability, and global competitiveness in the agricultural sector.
In the United States, biotechnology has revolutionized agricultural practices, such as, increasing crop production and variety diversification (Grossman, 2018). Along with other industries, the agricultural sector is experiencing the most significant impact from the blessings of biotechnology, or, in other words, genetic engineering (GE) technology.
For over 10,000 years, humans have employed conventional methods to alter crops and animals according to their preferences and requirements (Razzaq et al., 2021; U.S. FDA, 2023a). Examples of conventional methods for making these changes include cross-breeding, selective breeding, and mutation breeding. These breeding methods frequently entail combining the genes from two distinct sources (IAEA, 2022; Lee et al., 2015). Scientists trialed these methods to develop familiar crops, for instance, modern corn varieties and seedless watermelons (Thompson, 2023; U.S. FDA, 2023b; Melissa & Schleiger, 2022; Wieczorek et al., 2012). Scientists now use genetic engineering technology to transfer beneficial genes, such as insect resistance or drought tolerance, into plants with the blessing of this modern technology (Talakayala et al., 2020, Martignago et al., 2020, Gatehouse, 2008, Low et al., 2018, U.S. FDA, 2024a). In recent years, genome editing technologies have emerged as a powerful tool in agricultural biotechnology, facilitating the generation of innovative crop varieties devoid of foreign DNA integration. In contrast to conventional genetic modification that entails transgene insertion, techniques such as CRISPR/Cas9, TALENs, and ZFNs provide accurate modifications of an organism's pre-existing genetic material (Malzahn et al., 2017). This accuracy facilitates the overexpression or repression of particular genes to cultivate desired phenotypes while removing foreign sequences (Saravanan et al., 2022). Patel-Tupper et al. (2024) emphasized the advancement of disease-resistant crops using genome editing, particularly enhancing bacterial wilt resistance in tomato plants. Hayes (2023) illustrated in Nature Biotechnology that genome editing may improve maize's drought resilience by altering genes associated with water stress response. Cisneros et al. (2023) illustrated the efficacy of genome editing in enhancing rice yield and nutritional quality in their article in Nucleic Acids Research. The capacity to develop genetically modified crops devoid of foreign DNA has considerable benefits for public acceptance, since traditional GMOs encountered resistance stemming from apprehensions over foreign gene insertion and its long-term consequences (Aziz et al., 2022). Genome editing can produce comparable or superior outcomes while alleviating these issues, potentially resulting in diminished regulatory restrictions and more consumer acceptance (Callahan, 2023). The objectives behind genetic modification remain consistent throughout history: enhancing crop yields, reducing crop loss, extending storage life, improving appearance, boosting nutrition, or a combination of these beneficial traits (Babe, 2022, Butler, 2023, Ramos et al., 2023). We also expect the application of genetic engineering (GE) and genetically modified organisms (GMOs) in agriculture to enhance resistance to diseases and pests, and secure food safety and availability (Hossain & Roslan, 2023).
Despite its potential, biotechnology in agriculture has been the subject of vigorous debate and scrutiny. Particularly, widespread skepticism (Fig. 1) (Pew Research Center, 2020) with GMO safety, environmental effects, and ethical implications are at the core of these arguments (Abushal et al., 2021; EFSA, 2011). Research indicates that 32% of Americans with high knowledge of science and research believe that researchers often tend to favor industries based on their research outcomes related to GM foods (Funk & Funk, 2024). Moreover, when biotech crops are close to related plants, whether they are weeds or wildflowers, they have the ability to exchange traits with each other through pollen (Ellstrand, 2003; Warwick et al., 2009; Poppy, 2004; Snow, 1997; Martínez-González et al., 2021). The assessment of genetically engineered organisms also considered the potential environmental effect on birds, mammals, insects, worms, and others, especially when it comes to insect or disease resistance traits (Naranjo, 2021; USDA, n.d.; Ghimire et al., 2023; Wei & Stewart, 2023).
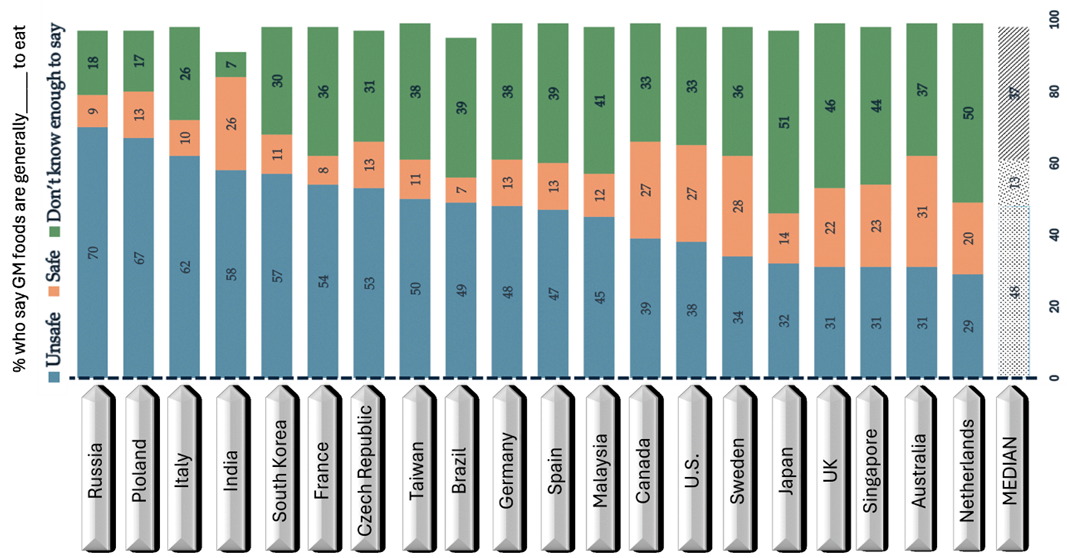
Figure: 1: Widespread skepticism about the safety of GM foods. Note that the respondents who did not give an answer are not shown. The data collected from the International Science Survey 2019-2020. Q20. (Pew Research Center, 2020)
On the contrary, GMO foods are equally healthy and safe as their non-GMO counterparts (Sims, 2020; Hollingworth et al., 2003; Teferra, 2021; Ghimire et al., 2023; U.S. FDA, 2023b). Basically, scientists have modified certain genes to improve their nutritional and dietary values. For instance, GMO soybean oil is healthier and can be used as a replacement for oils that contain transfat (Ghimire et al., 2023; Teferra, 2021; Shen et al., 2022). Studies have shown that GMO foods introduced in the 1990s, and are as safe as non-GMO counterparts (Shen et al., 2022; Klümper & Qaim, 2014; Bawa & Anilakumar, 2012). Moreover, studies indicate that GMOs used in animal feed are equally safe compared to non-GMO animal food (Norero, 2022; Shen et al., 2022; U.S. FDA, 2024b).
To address these challenges, regulatory frameworks play a key role in ensuring biotechnological progress, whether or not it adversely affects the environment or human health (Rozas et al., 2022, Tsioumani, n.d.). The U.S. Department of Agriculture (USDA), Food and Drug Administration (FDA), and Environmental Protection Agency (EPA) are the principal regulatory agencies in the United States responsible for GMOs and biotechnology regulations.
The study aims to assess the scientific foundations, future directions, and regulatory challenges of biotechnology in American agriculture. We will review the current regulatory framework to find shortcomings and propose modifications for sustainable integration. We will also look at the scientific foundation of biotechnology, assessments of health and safety, and effects on consumers' and growers' socioeconomic situations. The study will also review EPA, FDA, and USDA's regulatory measures and offer evidence of the security and efficacy of modified species. Our goal in tackling ethical concerns and the broader consequences of biotechnological developments is to provide insightful analysis and suggestions to the scientific community, industry stakeholders, and policymakers
This review investigates agricultural biotechnology operations in the United States by examining peer-reviewed articles, regulatory documents, and key stakeholder perspectives. The literature search spanned from 1981 to March 2024, using databases such as Google Scholar, Sci-Hub, Pub-Med, Agricola for peer-reviewed articles and official documentation from the USDA, FDA, and EPA. In addition, relevant data were retrieved from reliable websites related to (agricultural) biotechnology regulation and policy. Boolean operators (AND, OR, NOT) were used to refine the search.
Inclusion criteria: consisted of studies focused on US agricultural biotechnology (mostly GM crops), regulatory frameworks, safety assessments, and socioeconomic impacts.
Exclusion criteria: removed unreliable sources and studies irrelevant to these topics. Articles were selected based on their relevance, and duplicate studies were removed using citation management tools, Endnote.
Semi-structured interviews were conducted with extension associates, field officials, and industry experts to gain practical insights into the regulatory landscape and its real-world implications.
One of the main challenges to the seamless incorporation of biotechnology and genetically modified (GM) crops into the American agricultural system would be the intricate regulatory complexity and the fragmentation of regulations. As there is no dedicated legislation, the arrangement instead consists of borrowed portions of the existing laws, which makes the whole regulatory framework a very complex. While the Coordinated Framework established in 1986, U.S. Department of Agriculture (USDA), Food and Drug Administration (FDA), and Environmental Protection Agency (EPA) divided the work under this “Coordinated Framework for the Regulation of Biotechnology” (Martin, A., 2022). Although this framework has functioned for decades, some researchers have pointed out potential gaps or overlaps in regulatory oversight, particularly when it comes to novel biotechnologies like genome editing (McHughen & Smyth, 2007; Montpetit, 2005). To improve the efficiency of the system, there may be a need for better coordination and streamlining between these regulatory bodies, especially as new biotechnologies continue to emerge.
The main challenges in biotechnology include resource allocation and regulatory effort prioritization. Regarding risk-based practice, there is a recognition of the need to align regulatory resources with risk levels. But still, the question arises as to whether the existing resource allocation practices are enough. The scientific and regulatory community generally agree on the mechanism of "product, not process" regulation, but further research is necessary to determine whether current legislation can effectively regulate biotechnology and determine its positive or negative impact on regulatory efficiency.
The US and Canada, in contrast to Europe, did not pass any new laws pertaining to the regulation of biotechnology. Instead, existing statutes were adapted to encompass new products and technologies. This strategy was driven by the idea that the nation already had enough human and juridical power in the appropriate agencies to ensure proper protection of the environment and society (Montpetit, 2005).
The Food and Drug Administration (FDA) published a policy statement in 1992 that described the safety and assessment procedure for foods made from novel plant kinds, including those made using the technique known as recombinant DNA (Federal Register Announcement of May 6, 1992,” Kok et al., 2008). Since the first genetically modified crops were approved in 1994–1995 (U.S. FDA, 2023a; Rangel & Maurer, 2016), they have evolved and remain consistent with the oversight framework established by the U.S. Department of Agriculture (USDA).
4.1 USDA Oversight
Under a permit system, the USDA's Animal and Plant Health Inspection Service (APHIS) regulates managed field trials of prohibited genetically modified organisms and veterinarian products (Purchase, 1990). Meat and some poultry products are subject to safety regulations by the Food Safety and Inspection Service (FSIS), a division of the USDA (Brougher, 2011; Manchester et al., 1997). The genetic engineering method is the main emphasis of the process-based regulatory process at the USDA, which calls for permits and oversight for field testing of genetically modified organisms (Hoffman, 2021, McHughen, 2016). However, USDA regulatory mandates can easily be limited in scope to only environmental factors, resulting in whole categories of risk falling outside of regulatory scrutiny (Secchi, 2023).
4.2 FDA Authority
The FDA assures that genetically modified foods are safe for human and animal consumption. Under the Federal Food, Drug, and Cosmetic Act, the FDA evaluates food from GM crops with specific concern for allergens, toxicants, and the nutritional equivalence of the foods (Shen et al., 2022). Specifically, the Centers for Food Safety and Nutrition (CFSAN) and the Center for Veterinary Medicine (CVM) of the FDA, in particular, evaluate the safety of genetically modified crops, milk, and dairy products (Jarrell et al., 2015). In contrast to a process-based approach, the FDA evaluates the qualities and attributes of the food before imposing regulatory scrutiny based only on the biotechnology process. FDA connection is discretionary, but if harm becomes apparent, not participating could lead to a recall that is required and legal action (Tennyson, 2011). However, the lack of legal restrictions on the recommendations of the FDA to engage in a pre-commercial consultation results in wide criticism, with apportionment of allowances to GM food developers, while their compliance with the guidelines is purely voluntary. These regulatory lapses add to both fragmentation and public fear of the processes involved, which in turn contribute to minimal transparency (Naveen & Sonatakke, 2024).
4.3 EPA Involvement
The EPA is in charge of regulating chemical insecticides and herbicides and oversees the evaluation of genetically modified plants that have pesticidal, Plant-incorporated Protectant (PIP) genes incorporated into them (Murphy & Krimsky, 2003, Ehn RC, & Fox JR, 2019). The EPA basically follows two Acts-the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) and the Toxic Substances Control Act (TSCA). Among other regulatory functions, the agency assesses potential environmental impacts of pest-resistant crops, for instance, Bt corn, to ensure that those plants do not pose unintended risks to non-target species and the ecosystem. While EPA is focuses on the environmental impacts of GM crops, it does not look into larger issues of agricultural management or food safety, which fall under USDA and FDA, respectively (EPA, 2023). Moreover, FDA and EPA both adhere to identical standards for food safety. A thorough dossier must be submitted to the EPA and discussions with the FDA are part of the PIP evaluation process (McHughen & Smyth, 2007). Mandatory USDA and EPA requirements guarantee a rigorous review process (Spector, 1975).
| Agency | Primary Responsibilities |
| USDA | It regulates the introduction of genetically engineered organisms(USDA (n.d). |
| Assesses environmental impacts and manages field trials (USDA-APHIS (n.d.) | |
| FDA | The FDA operates under the Federal Food, Drug, and Cosmetic Act (FFDCA). It oversees the safety of food and feed products derived from GMOs (US FDA, 2023). |
| EPA | The FFDCA evaluates safety and nutritional aspects (US FDA, 2023). |
| Regulates pesticides produced by genetically modified plants (US EPA (n.d). | |
| evaluates environmental impacts and effects on non-target organisms (US EPA, 2024). |
Table 1: Key regulatory responsibilities of each agency
(As of 2023) 645 GM crop events are listed by the Center for Environmental Risk Assessment, taking into account several breeding techniques. GM approval data, including additional crop varieties, is kept up to date in a database maintained by the International Service for the Acquisition of Agri Biotech Applications (ISAAA.org, n.d.). To ensure accessibility, every regulatory body-including the EPA, FDA, and USDA-maintains distinct databases for each of their decisions about specific GM incidents (Freese & Schubert, 2004). Most genetically modified crops (GM) occur in commodity crops such as soybean, canola, cotton, and maize. In 1996, the genetically modified (GE) seed was first commercially introduced in the United States as a major field crop. The United States has seen a remarkable adoption rate of genetically modified crops; in 2014, over 90% of planted maize and soybeans were GM varieties (Fernandez-Cornejo et al., 2014). Since the commercial launch in 1996, their (GM seeds) adoption rate has increased rapidly. Later, GM crops, mostly canola and sugar beets, were broadly adopted. By 2024 (the most recent year for which data are available), approximately 55% of the total harvested cropland was cultivated with at least one GM trait (Figure. 2) (USDA-ERS. n.d.).
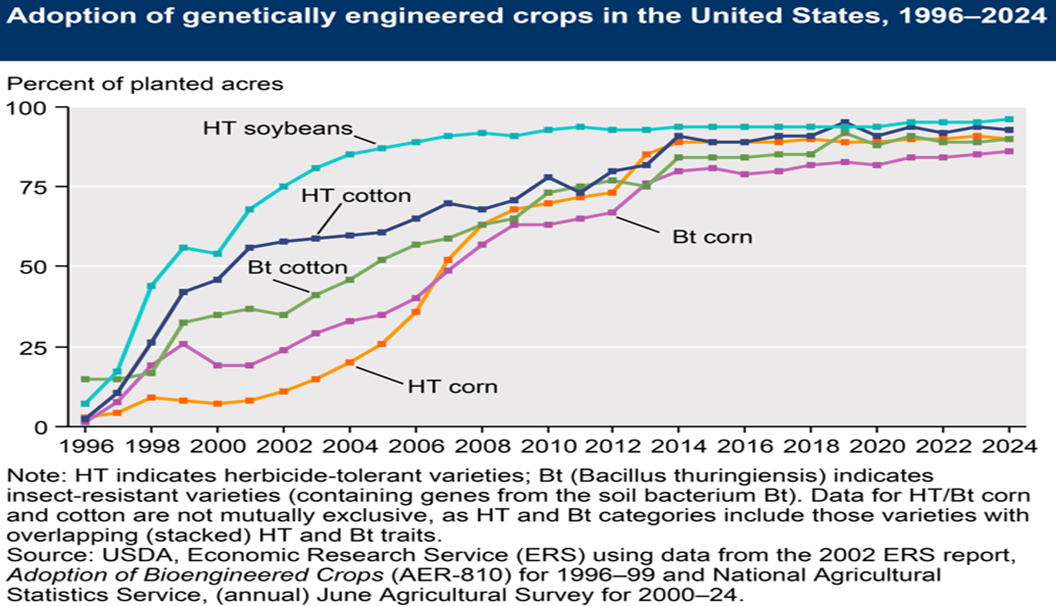
Figure 2: Recent trends in genetic engineering adoption
Herbicide tolerance and insect resistance are most common (Table 2) (USDA-ERS. n.d.). For detailed information, the table 2 can be viewed on the USDA ERS website here (Accessed October 10, 2024).
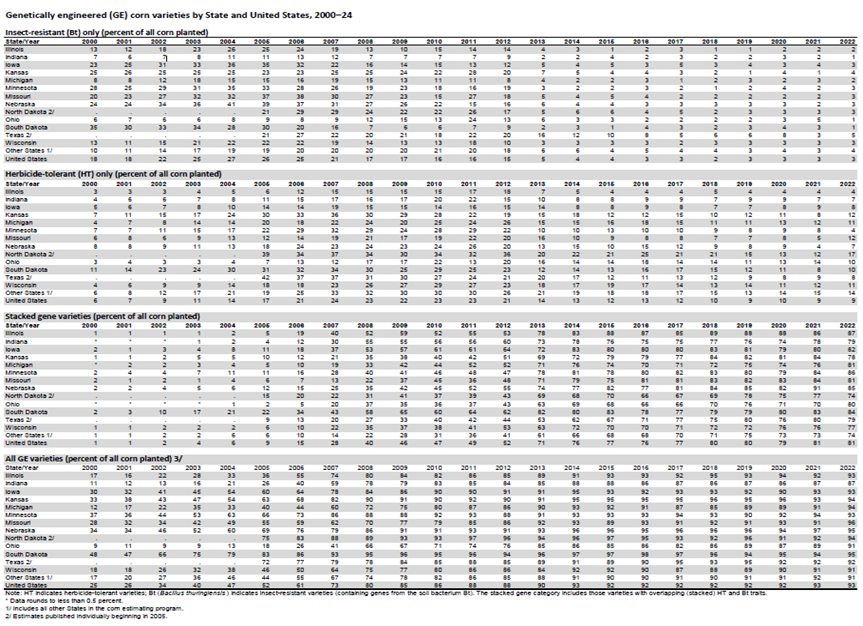
Table 2: The data provides an overview of the adoption of herbicide-tolerant and insect-resistant corn, cotton, and soybeans, by State and for the Unites States, 2000–24.
The field of genetically modified crops has evolved, with many GM events serving comparable purposes. Some genetically engineered crops were removed due to pressures from customers and businesses, but others-e.g., viral-resistant papaya-have efficiently addressed certain issues and saved the corresponding industries (Kour et al., 2022). Fresh GM crop sweet-sorghum participated in a fast-paced adoption in China, India, and the United States becoming a major portion of the worldwide production of cotton and entering the market (Raman, 2017). Since the introduction of GM crops, they have consistently contributing in global economic gain. Their cumulative economic contributions are substantial (Figure. 3) (Chaudhary & Singh, 2018). In particular, insect-resistant (IR) maize and IR cotton stand out with the highest cumulative economic benefits, with IR maize alone contributing approximately $33.40 billion and IR cotton contributing $11.10 billion. According to the recent reports, GM crops produced an estimated $261.3 billion in economic benefits from 1996 to 2019 (ISAAA - AFRICENTER, 2019). Moreover, before marketing and consumer access, GM products have to undergo strict safety and regulatory processes.
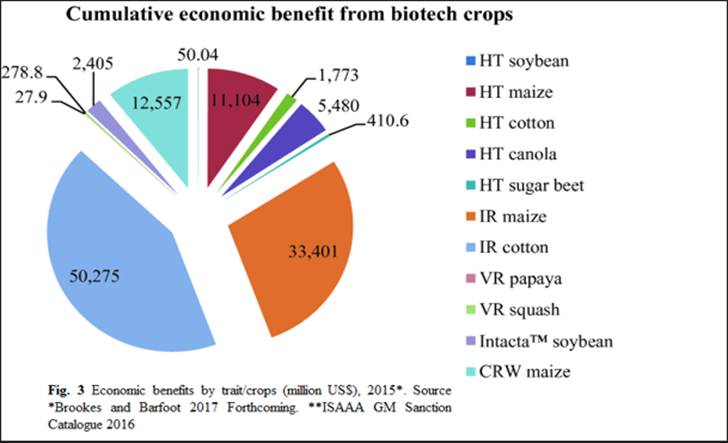
Figure 3: Economic benefits by trait/crops (million US$), 2015. The data sources are the Brooks and Bafoot 2017 Forthcoming and the ISAAA GM Sanction Catalogue 2016 (Chadhary & Singh, 2018)
7.1 Socio-Cultural Factors
Public skepticism towards GMOs often arises from socio-cultural and ethical concerns. Many individuals express discomfort with the "unnaturalness" of genetic modification that goes against the sanctity of nature. These viewpoints are grounded in long-standing agricultural practices and cultural norms favoring organic, indigenous varieties of crops (Hund & Wald, 2020). Certain religious and ethical perspectives condemn the act of altering or tampering with the natural phenomena of life or disrupting the natural order of lives, guided by the belief that individuals should work in harmony with nature, rather than try to manipulate it (Hund & Wald, 2020). Misinformation and lack of clear communication of the technology can further increase these concerns.
7.2 Economic Factors
Skepticism towards GMOs is also rooted in economic issues, particularly in the developing world. For instance, small-scale farmers may object to GMO adoption, as multinational agribusinesses aggressively market and sell GMO seeds while exercising rights over the intellectual property of their seeds. These farmers cannot afford to pay for the high cost of GM seeds, which they may fear could eventually tie them to corporate-controlled farming practices (Naveen & Sontakke, 2024; Sandhu et al., 2024). Additionally, some consumers believe that GMOs primarily benefits agribusiness, rather than address global hunger or improve farmers' livelihood (Sandhu et al., 2024).
7.3 Political and Regulatory Factors
The politics of governing GMOs also serves as an important determinant of the public opinion. Citizens in several countries, and groups that represent them, are opposed to GM crops (Naveen & Sontakke, 2024; Turnbull et al., 2021). This is especially true for anti-GMO movements created by political parties, environmentalist groups, or consumer rights groups who demand that GM products be avoided or demand that the use of the technology be restricted or banned. Much of this pressure has effectively limited the technology's use within several members of the European Union (Bain & Dandachi, 2014). Inconsistencies in regulatory policies across the world too has lent a hand in fueling the apprehension surrounding GM crops. Although the United States uses a fairly liberal policy, EU, in the wave of popular anti-GM sentiment in its member countries has set in place regulations that leaves relatively less freedom for GM crops to be developed and used in its members. This fragmented policy contributed to the divergence of perception over the safety and wisdom of utilizing GMOs (Naveen & Sontakke, 2024; Turnbull et al., 2021).
8.1 Education
Educational outreach should focus on increasing public awareness of demystifying GMOs by providing clear, reliable information regarding the science of genetic modification. The targeted public awareness campaigns should be grounded on science-based accounts of the safety procedures and regulatory framework regarding the production and consumption of GMOs and combat common myths (e.g., that GMOs are inherently unsafe) (Cornell Chronicle, 2022). In addition, infographics and forums hosted in local communities to establish a two-way conversation among scientists and communities will encourage members of the community to raise their questions and concerns (Biosafety Information Centre. (n.d.). Infographics can also be launched to the supermarket shelves or labels to inform consumers of the importance of re-examining the acceptance of GMOs and the benefits it may have for both the food security and environmental sustainability of the nation (Verma, S., 2024).
8.2 Engage with Cultural Values
Educational effort targeting different cultural groups should also ensure that the sensitivity of the region is properly maintained and that important ethical or religious concerns are taken into account. This might involve partnerships with community leaders, religious leaders, or public figures who are well-respected in the community. Community-based participatory research process also helps ensure that the voice of small farmers and vulnerable community members is heard as well. This makes sure that critical ethical and religious concerns are understood and that the citizens of that country understand the benefits of GMOs to the food security and environmental sustainability (Naveen & Sontakke, 2024; Fan et al., 2021).
8.3 Emphasizing the Role of Genome Editing
Genome editing, particularly the CRISPR-Cas9 approach may serve as a more acceptable approach for biotechnological development. Unlike traditional genetic modification where foreign genes are often inserted, genome-editing allows for the removal and editing of the genes to be modified without the insertion of foreign material (Javaid et al., 2022; Reardon, 2023). This may mitigate fears of "unnaturalness" of using foreign material in food. Educational efforts can point out the role and importance of genome editing to its ability to improve aide the crops in climate resilience, increased nutritional content, and reduce pests while not changing the crop’s genetic identity (Ronald, P. & Cliegman, M., 2024). Scientists must especially point to the rigorous standards and principles that the product had to meet to gain its regulatory approval standards, and regulatory principles and safety standards which is often more streamlined than that for GMOs (Chaparro, T., 2024).
In addition, targeted social media campaigns can effectively promote the safety, advantages, and possibilities of GMOs and genome editing, engaging younger audiences via concise informative videos. Integrating biotechnology and genetic engineering into the school curriculum helps enhance scientific literacy and critical thinking. What’s more, collaborations among governments, NGOs, and biotechnology firms can demonstrate practical instances of GMOs and genome-edited crops tackling food security, climate resilience, and nutritional deficiencies. These activities can transform the discourse from apprehension to optimism over the prospective advantages of biotechnology for society (Gene-Editing, 2024).
The inception of recombinant DNA technology in the early 1970s in the United States prompted immediate concerns about potential risks associated with genetic engineering (Bur & Wright, 1996). Scientists raised apprehensions, leading to the Asilomar Conference in 1975, where discussions addressed various risk scenarios related to recombinant DNA technology (Krimsky, 2005). In response, the National Institutes of Health (NIH) published the guidelines for research involving recombinant DNA molecules in 1976 (NIH, 1987; Singer, 1977). While not codified into law, laboratories receiving federal funds adhered to them. Other labs, both public and private, voluntarily followed these guidelines, recognizing their prudence.
Certain limitations were eased as time passed on and the scientific and regulatory institutions gained greater familiarity with the technology and its offerings. When it was suggested that organisms with gene editing might be unleashed into the environment in the early 1980s, public opinion evolved (Devos et al., 2007).
The White House Executive Office organized an interagency working group to deal with these concerns, which examined the veracity of the allegations and suggested a regulatory strategy. The result was the 1984 draft of the "Coordinated Framework for Regulation of Biotechnology," that was finalized by the White House Office of Science and Technology Policy (OSTP) in 1986 (Shapiro & States, 1989; OSTP, 1986). This paradigm yielded several important findings (summarized from Payne & Medley, 1992) that will direct science and policy going forward:
The laws that are now in place give sufficient jurisdiction to regulate biotechnology products. Amazingly, it is believed that these foundational legal and regulatory safeguards were foreseen about 50 years ago, even before any genetically modified organisms were released or ingested by humans (Freese & Schubert, 2004).
In 1992, the Office of Science and Technology Policy (OSTP) released a position statement to strengthen the scientific foundation. This declaration stressed a risk-based, scientifically sound approach to biotechnology products, placing a greater priority on the traits of the product and how it impacts the environment than on the method of generation (Freese & Schubert, 2004).
One of the main concerns surrounding GMOs is the possibility of unforeseen consequences, particularly those that may pose a risk. Thorough evaluations conducted by scholars, including the 2004 report from the United States National Academy of Sciences and the Institute of Medicine, provide a detailed analysis of the risks involved in plant breeding (Kessler & Economidis, 2001; NRC, 2004). It compares contemporary genetic modification techniques and those employed in earlier times. Unintended consequences are the main focus, seen as indicators of unforeseen risks. Modifying the DNA of a plant, animal, or microbe can have unforeseen consequences, potentially resulting in unintended or risky traits. including gene flow and cross-contamination, which may adversely affect the environment. An illustrative instance is Star Link Corn, a GMO-approved-only animal feed found in human food products in 2000, exacerbating public concern regarding the inadvertent dissemination of GM crops. A notable case is the herbicide-resistant Brassica napus (canola), wherein transgenes escaped wild relatives, resulting in the emergence of herbicide-resistant weeds, a phenomenon termed transgene escape, which complicates the management of invading species (Shen et al., 2022; Sabat & Tripathy, 2024). It is proven that genetic modification may influence the biochemical pathways in ways that are not predictable, resulting in unexpected changes, e.g., producing new metabolites that were not present in the original organisms (Sorochinskii, et al., 2011; Zheng et al., 2020, Goyal et al., 2021), which may adversely affect the environment. However, the standard breeding process successfully identifies and eliminates undesirable traits before introducing them to the market. Modern cultivars have been specifically engineered to thrive in optimal farming conditions, effectively minimizing the chance of undesirable traits (Benavente & Giménez, 2021; Sun et al., 2024).
The breeding techniques used in livestock, fiber, food, and industrial applications have a long history of safety, with only a few alleles causing harm (Bishop & Van Eenennaam, 2020; Dekkers, 2019). The infrequency of unfavorable outcomes among many new varieties highlights the general security of breeding methods. Although every breeding technique has its own potential effects, a comprehensive evaluation process has proven successful in identifying and minimizing any risks involved (Zilberman et al., 2018).
Naturally occurring toxicants found in food and feed plants include mycotoxins in cereals and glycoalkaloids in potatoes. Researchers are constantly developing plant cultivars free of these dangerous chemicals (Kumar et al., 2018, Hansen et al., 2021, WHO, 2023). One obvious sign of the attempts made to lessen or completely eradicate the presence of toxic compounds is the ongoing work to selectively breed plants (Asghar et al., 2024). Many aspects of human existence can be significantly disrupted by toxins and allergies. From mild coughing to a runny nose to severe symptoms including anaphylaxis (Cleveland Clinic, 2020), allergies trigger off immunological reactions. Toxins can throw off cell activities, induce inflammation, oxidative stress, and damage to key organs (Hoag, 2024). Toxins including heavy metals and mycotoxins can damage kidney performance (Ding et al., 2023) by creating oxidative stress and damaging renal cells). Long-term exposure to some toxins (BiologyInsights, 2024) can cause proactive kidney damage and diseases like chronic kidney disease (CKD). Generating DNA changes (Ding ewt al., 2023) allows various toxins—including aflatoxins—to be carcinogenic and increase a cancer risk. On the other hand, allergies have a complex relationship with cancer; some studies indicate a probable risk resulting from chronic inflammation (Cleveland Clinic, 2020), while others reveal a preventive role resulting from better immune monitoring. Allergies induce the immune system to react to innocuous molecules, which generates immunoglobulin E (IgE) antibodies and generates histamines (Cleveland Clinic, 2020). Toxins can lower immune system activity, therefore raising the body's susceptibility to diseases and infections (Hoag, 2024). By means of several pathways—DNA damage, protein synthesis inhibition, oxidative stress, and immunological suppression—mycotoxins produce their toxic consequences (BiologyInsights, 2024). Although totally getting rid of allergies and toxins is still a difficult task, plant breeders have made use of conventional breeding methods (Khan et al., 2020). Notably, in comparison to breeding methods, the growing environment frequently has a greater impact on the inherent hazards associated with food (NAP 20024; Jiang et al., 2021). Rarely do conventionally bred crops produce unexpected effects, such as the Lenape potato, which under some circumstances can have high selinene levels (Koerth-Baker, 2013; Bradshaw, 2019). Conventional breeding methods have, nevertheless, been continuously shown to be rather safe (Thrupp 2000; Kaiser et al., 2020).
Contrary to popular belief, both conventional breeding techniques and genetic engineering can transmit genes between various species. Induced mutation, one of the modern breeding methods, has a continuously safe history (Parrott, 2010). Observations highlight the significant role of unintentional genetic impacts, underscoring the challenge of distinguishing between safe and dangerous breeding techniques. Recombinant DNA (rDNA) technologies are one of the breeding strategies that, according to Berg et al. (1975), presents fewer hazards in relation to serious health and safety issues. More research has confirmed that, in comparison to other breeding techniques, rDNA does not cause the genome to mutate more significantly. This confirms the general agreement that breeding methods ought not to be interpreted as justifications for regulatory action (McHughen, 2016; Anderson et al., 2016)
To alleviate these risks, numerous detection and preventative measures have been developed. Molecular markers, like Simple Sequence Repeats (SSRs) and Single Nucleotide Polymorphisms (SNPs), are essential for detecting gene flow (Taheri et al., 2018). SSR markers have been successfully utilized to monitor gene flow from herbicide-resistant canola (Zhang et al. 2023), facilitating prompt interventions to avert further spread.
Alongside detection, measures for containment have been developed to execute gene flow. A conventional approach is an isolation or physical distance, which entails cultivating GM crops considerably from non-GM or wild varieties to reduce pollen dispersal (Price & Cotter, 2014). This approach has been used in Mexico to protect indigenous maize from contamination by GMO maize (Hu et al., 2022). Additional advanced strategy involves biological containment, such as Genetic Use Restriction Technologies (GURTs), which are commonly referred to as ‘terminator technology.’ GURTs assist prevent the unintended spread of transgenes through natural reproduction by ensuring that the seeds of GM crops are sterile after the first generation (Lombardo, 2014). By combining such approaches with regular molecular marker monitoring, a strong barrier against the unforeseen ecological effects of GM crops is created (Clark & Maselko, 2020).
The protection of our environment, community, and society as a whole is the goal of the regulatory bodies. Ideally, hazards to life, food and feed security, and ecosystems would all be eliminated by carefully targeted rules and efficient risk management strategies (Hasnas j., 2009, Pacheco-Vega, R., 2020). But to control and manage certain possible dangers, it is necessary to allocate human, financial, and temporal resources wisely for pragmatic reasons (US EPA, 2020). No nation can really control every facet of every potential threat in its entirety. As a result, a system of priority needs to be applied everywhere. The primary cause of the variances seen in this study between various jurisdictions is the differing priority policies and procedures.
Effective prioritization necessitates a careful evaluation of the risks connected to different hazards and concentrating resources on managing and reducing risks from those that pose the biggest danger.
Recognizing the merits of the 'product, not process' approach to biotechnology product regulation is crucial. Furthermore, it is critical for the United States to demonstrate that biotechnology regulation can be accommodated within the framework of currently enacted legislation, even if it needs modification or interpretation with some flexibility to properly address possible targets (McHughen, 2016).
McHughen (2016) also emphasized the significance of establishing strong working ties with various departments and agencies and creating appropriate Memorandums of Understanding (MoU), which allowed for logical cooperation in carrying out necessary tasks. Further enhancing the process is the idea of a "single desk" or "one-window shopping," in which a proponent applies to one department or agency, and that agency coordinates with any other agency to complete the regulatory evaluation for the product.
A regulatory shortage also occurs when hazards are not sufficiently identified and regulatory resources are not allocated in a manner commensurate with the amount of risk. As a result, mistakes are made in both commission and omission: comparatively higher-risks are under regulated, while lower-risk ones are overregulated. In addition to wasting resources, misallocating resources to address lower-risk issues exposes the environment and public health to possible harm from higher-risk but less obvious concerns (US FDA-Office of the Commissioner, 2024).
In order to successfully regulate and preserve public faith in the regulatory mechanism, it is obvious that effective prioritization and coordination are essential (US EPA 2016).
Having a clear understanding of biotechnology and genetically modified organisms (GMOs) in American agriculture is the first step in considering their potential impact on health and the environment. Without a doubt, strong legal and policy implementation through collaborative action and communication among the agencies, working towards a common goal with one another, can ensure the safe and sustainable development of this area. Aside from that, the specific creative approaches that enable biotechnology may focus on the good of people, localities, and the planet. We can achieve this by acknowledging the challenges highlighted in this study and implementing the measures suggested.
Not applicable
Not applicable
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
The authors declare that they have no competing interests
This research is supported by the Agriculture/Agricultural Regulation, University of Arkansas at Pine Bluff, and USDA, Grant # 100174.
ORCHID: 0000-0003-3131-6692 (Shahidul Islam)
MSR summarized the data, organized the manuscript, and was a major contributor to writing the manuscript. SI organized and mentored the research, reviewed the manuscript, provided suggestions, and supervised all over the project. MAKA reviewed the manuscripts and provided suggestions. All authors read and approved the final manuscript.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti
Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation. “The peer review process was efficient and constructive, and the editorial office provided excellent communication and support throughout. The journal ensures scientific rigor and high editorial standards, while also offering a smooth and timely publication process. We sincerely appreciate the work of the editorial team in facilitating the dissemination of innovative approaches such as the Bonori Method.” Best regards, Dr. Matteo Bonori.

I recommend without hesitation submitting relevant papers on medical decision making to the International Journal of Clinical Case Reports and Reviews. I am very grateful to the editorial staff. Maria Emerson was a pleasure to communicate with. The time from submission to publication was an extremely short 3 weeks. The editorial staff submitted the paper to three reviewers. Two of the reviewers commented positively on the value of publishing the paper. The editorial staff quickly recognized the third reviewer’s comments as an unjust attempt to reject the paper. I revised the paper as recommended by the first two reviewers.
