AUCTORES
Globalize your Research
Review ariticle | DOI: https://doi.org/10.31579/2690-1897/254
Department of Pharmacology, School of pharmacy, RK University Rajkot, India.
*Corresponding Author: Kiran Dudhat, Department of Pharmacology, School of pharmacy, RK University Rajkot, India.
Citation: Chinmyee Saha, Ishita Zalavadiya, Kiran Dudhat, (2025), Emphasis on the In-Vivo & In-vitro Screening Models for the Evaluation of Disease Acting on Alzheimer’s Disease, J, Surgical Case Reports and Images, 8(5); DOI:10.31579/2690-1897/254
Copyright: © 2025, Kiran Dudhat. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 08 May 2025 | Accepted: 19 May 2025 | Published: 27 May 2025
Keywords: neurodegenerative disorder; in-vitro model; in-vivo model; evaluation; screening
Alzheimer's disease is a neurodegenerative disorder with cognitive impairment and pathological hallmarks. Alzheimer’s disease causes the brain to shrink and brain cells to eventually die. Alzheimer’s disease is the most common cause of dementia. Therapeutic interventions are challenging, and in vivo and in vitro screening models are valuable tools for evaluating drug candidates. These models evaluate cognitive performance, neuropathological changes, and molecular biomarkers, providing a comprehensive understanding of drug efficacy. In vivo models, like transgenic mouse models with Alzheimer's disease genetic mutations, allow researchers to study drug effects within a complex biological system, evaluating cognitive performance, neuropathological changes, and molecular biomarkers. In vitro models, like cell-based assays, neuronal cultures, and organoids, provide insights into drug mechanisms, toxicity profiles, and selectivity, and facilitate high-throughput screening for large compound libraries and potential lead molecules. The review article highlights the significance of in vivo and in vitro screening models in drug discovery and development, particularly in preclinical research for evaluating Alzheimer's disease drugs. In vivo and in vitro screening models for Alzheimer's disease drugs have significantly enhanced understanding and therapeutic interventions, aiding in screening compounds, assessing efficacy, and optimizing lead candidates for further research.
Alzheimer's disease is a progressive neurodegenerative disorder affecting around 50 million people worldwide, causing a decline in memory, executive function, and personality change. It is named after Alois Alzheimer and results in synapse loss and neuronal atrophy, characterized by amyloid plaques and neurofibrillary tau tangles. Both genetics and environmental factors are believed to play a role in AD, with most cases being sporadic and having no single genetic cause. Environmental and metabolic risk factors include diabetes, cerebrovascular disease, poor diet, head injury, and stress. The amyloid hypothesis, which suggests AD begins and progresses, leaves many questions, including the best drug target and the upstream cause of the rise in amyloid-β in sporadic cases. There is still a lack of understanding of how AD develops and therapies to help individuals combat the disease [1-7]
1.2. Types of Alzheimer's Disease
Early-onset Alzheimer's (EOA)
Those under65, frequently in their 40s or 50s, are susceptible to early-onset Alzheimer's. As little as 5% of all cases of Alzheimer's disease demonstrate this rarity. It is more prevalent in those who have Down syndrome and is connected to changes in the brain like plaques, tangles, and loss of volume. [8]
Late-onset Alzheimer's (LOA)
The most prevalent type of Alzheimer's disease, known as late-onset Alzheimer's (LOA), affects individuals 65 years of age and older and has no known genetic cause or family history [8]
Familial Alzheimer's disease (FAD)
A genetically linked form of Alzheimer's disease, familial Alzheimer's disease (FAD) affects at least two generations in affected families and accounts for less than 1% of cases overall [8]
1.3. Etiology
Aβ precursor protein
Genes exhibiting autosomal dominant mutations that cause Alzheimer's disease first appear in the Aβ precursor protein (APP). Ten percent of cases that are familial are caused by more than fifty known APP mutations, most of which are centered around β and γ-secretase cleavage sites. Numerous mutations, according to research, raise Aβ synthesis, which promotes amyloid buildup. Promoter mutations or APP duplications may in rare instances result in AD [9,10] .
Presenilin
Autosomal dominant AD is caused by the γ-secretase catalytic components encoded by presenilin 1 and 2. Thirty to fifty percent of cases of familial EOAD are due to variants in PSEN1. Based on research, mutations in PSEN1 and PSEN2 change the production of Aβ but result in a loss of function.
Other genetic risk factors
A gene's susceptibility to Alzheimer's disease is associated with variations in TREM2, APOE, CLU, BIN1, SORL1, and PICALM. The most frequent risk factor, the E4 allele of APOE, triples risk. The uncommon variation TREM2R47H contributes to inflammation in the pathophysiology of AD. [11-13]
Down syndrome
By the age of 65, dementia affects up to 80% of people with Down syndrome (DS) [14]. Like other cases of EOAD, even at a young age of less than 40, amyloid and tau pathology start considerably earlier than in LOAD [15–16]. when compared to LOAD, amyloid and tau pathology start sooner. Gene triplication may also contribute to DS, which is caused by chromosome 21 trisomy and elevated Aβ levels. [14, 18-19]
Inflammation
Brain hypoperfusion and inflammation are common in sporadic Alzheimer's disease (AD), which is a result of a combination of genetic and environmental factors [20, 21]. Traumatic brain injury and bone fractures increase the risk of dementia. A faster rate of cognitive decline is linked to higher levels of inflammatory markers as well as activated microglia and astrocytes in patients with AD. [23]
Cerebral, cardiovascular disease and diabetes
An elevated risk of Alzheimer's disease (AD) has been associated with vascular disease, including cardiovascular and cerebrovascular diseases [24]. Diabetes doubles the risk of dementia, and other lifestyle factors like obesity, high cholesterol, poor diet, and sedentary behavior also increase the risk. [25,27]
1.4 neuropathology
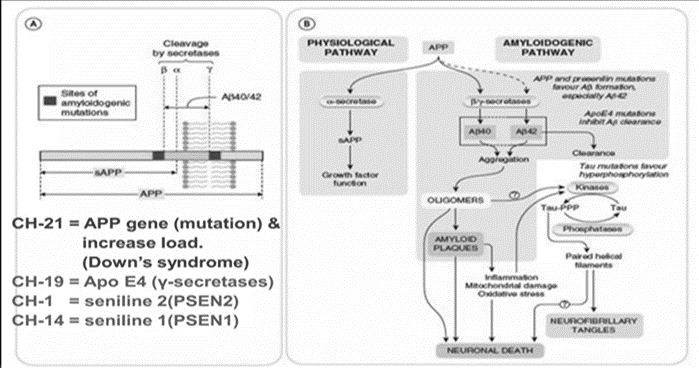
Figure 1: Alzheimer's Neropathology [28]
Aβ plaques
The brain is affected by senile plaques, which are composed of resistant Aβ sheets formed by amyloid peptides that co-localize with microglia and neuronal debris. In older people with normal cognitive function, they can be significant and come before tau. (29–31), but other research indicates
a link between dementia severity and cognitive decline. Perhaps a more accurate indicator of disease would be variations in the concentration of Aβ oligomers. (32-23). When Aβ pathology precedes cognitive changes, individuals with high plaque levels who are cognitively normal may have "prodromal" disease. (34, 35).

Figure 2: (A) Thioflavin S staining of Aβ plaques in the cortex of CRND8 APP transgenic mouse. (B) AT8 staining of NFTs within an aged human CA1 region- hippocampus. (C) Spread of amyloid and tau throughout the brain[36]
Neuronal fibrillary tau tangles:
Alzheimer's disease and other neurodegenerative diseases are associated with hyperphosphorylated tau protein aggregates, or NFTs. They diffused across the brain, producing toxicity and loss of neurons, but they had little association with deteriorating cognitive function. Confirmation and testing are necessary for diagnosis. [37,38]
1.4 Pathogenesis
Aβ and APP
Alzheimer's disease (AD) may result from mutations in the APP and presenilin genes, according to the amyloid hypothesis. The development, growth, and survival of neurons are influenced by both APP and Aβ, with the accumulation of Aβ possibly playing a role in AD. [39,40]
NFTs and Tau
A key player in neurodegenerative diseases such as Alzheimer's disease is the cytoskeletal protein tau. Neurotoxic effects from mutations can include altered axonal transport, transcriptional changes, and increased degeneration. Together, tau and Aβ result in transcriptional deficiencies and alterations to the synapses in AD. [41]
Mitochondrial dysfunction and oxidative stress
When mitochondrial function is disrupted by Alzheimer's disease, cytochrome oxidase activity is lowered, oxidative stress increases, and changes in mitochondrial morphology, number, and transport occur. These effects are both hereditary and environmental. While transgenic APP mice show increased Aβ within synaptic mitochondria, causing dysfunction and oxidative stress, Thy-1-APP mice show reduced membrane potential and increased ROS production [44]
Insulin
Oxidative stress and energy deficiencies can result from insulin resistance, reduced receptors in the Alzheimer's disease (AD) brain, and late-stage diabetes. Insulin is involved in neurotransmission and has the ability to shield the nervous system from damage [46]. In mice, levels of Aβ and β-secretase are elevated by insulin and metabolic inhibitors. [47]
Hypoglycaemia and vascular dysfunction
Alterations in metabolic proteins, glucose receptors/transporters, or hypoglycemia brought on by excessive medication may be the cause of the connection between diabetes and Alzheimer's disease. Tachycardia, increased oxidative stress, and neuronal death may result from this. [48]
Inflammation
Alzheimer's patients rapidly deteriorate as a result of inflammation, which has a significant impact on the disease. While it first shields neurons, it can also lead to tau pathology, increased Aβ load, neurotoxicity, and ROS levels. [49-52]
Ubiquitin-proteasome system
Through an enzymatic process, the ubiquitin-proteasome system (UPS) degrades excess and misfolded proteins, which is essential for synapse function. Targeting monomeric proteins may prevent proteasome activity[53], which could result in a hazardous accumulation of extra proteins in the brain.
Autophagy lysosome pathway
An involvement of autophagy in tau clearance and Aβ generation is suggested for AD pathogenesis, along with lysosomal dysfunction. BIN1, SORL1, and PICALM are among the genes linked to AD that may have a direct impact on APP endosomal processing. [55,56]
Cholinergic hypothesis
Linking acetylcholine with other pathologies is still difficult, but the cholinergic hypothesis—which was first proposed in AD—suggests aberrant levels of acetylcholine in the brain, affecting cholinergic neurons and affecting Aβ accumulation [57]. In areas where plaques and tangles are present, pyramidal neurons—which are mainly glutamatergic—are lost. [58].
1.5. Mechanism of Alzheimer Disease

Figure 3: Enzyme acts on APP (Amyloid Precursor Protein) 7 cut into in fragments. The beta amyloid fragments is crucial in the formation of sanile plaques in AD disease [59]
Aβ hypothesis:-
Neurotoxicity, senile plaques, and the clinical manifestations of AD are caused by increased production of Aβ fiber. Plaques are caused by the breakdown of APP into β-amyloid fragments by enzymes after it penetrates the neuronal membrane.
Tau hypothesis:-
Healthy neurons have microtubules, which act as tracks for nutrients and molecules. In Alzheimer's Disease (AD), tau, a protein, becomes tangled, leading to microtubule disintegration and collapsing the neuron's transport system, potentially causing communication malfunction and cell death.

Figure 4: Dendrite disinteration in Las protein tau [59]
1.6 Current treatment for Alzheimer disease
• Acetylcholine esterase inhibitors (AChEIs) [60]
Tacrine, Donepezil, Galantamine, Rivastigmine & Huperzine A
• NMDA receptor Antagonist [60]:
Memantine

Table 01: Details about current using drugs for AD [(60]
1.7 Limitation of conventional drugs available for Alzheimer Drug:
Alzheimer's medications, like cholinesterase inhibitors and memantine, provide temporary relief but do not address the underlying cause or slow its progression. Their effectiveness varies among individuals, with modest long-term effects. Current medications do not prevent or reverse Alzheimer's disease progression, and their use is limited to mild to moderate stages, with no FDA-approved medication for severe Alzheimer's.
1.8 Importance of drug development in Alzheimer disease:
Alzheimer's disease has no cure and current treatments provide temporary relief. Drug development is crucial due to the increasing number of affected individuals and lack of curative treatments. A curative treatment could improve the quality of life for millions by eliminating physical, emotional, and financial care. Drug development can also enhance our understanding of the disease, revealing its underlying causes and improving diagnosis and treatment precision.
1.9 Alzheimer’s drugs in development
Aducanumab:
Aducanumab targets beta-amyloid protein in the brain, causing AD symptoms by preventing messages from being sent between cells. [61]
Solanezumab:
Solanezumab, an anti-amyloid drug, is being studied for its potential to slow cognitive decline in individuals with Alzheimer's disease who do not yet show memory loss or thinking difficulties. [61]
Insulin:
The Study of Nasal Insulin in the Fight against Forgetfulness (SNIFF) is investigating if a nasal spray type of insulin can enhance memory function in individuals with mild memory issues or Alzheimer's disease. [61]
Others:
Current drugs include verubecestat, AADvac1, CSP-1103, and intepirdine, but AD is unlikely to be cured by a single medication, focusing on prevention and treatment. [61]
1.10Alternative treatments for Alzheimer’s disease
Coconut oil
Caprylic acid, found in processed coconut oil, is used in Ketasyn, a drug that improves memory performance and reduces cognitive decline [61]
Omega-3 fatty acids
Omega-3 fatty acids, found in fish, nuts, and oils, may reduce cognitive impairment. [61]
Coral calcium
Coral calcium, derived from seashells and sea life, is a popular calcium supplement for treating AD. [61]
Herbal medicine
Ginkgo biloba, an herb with anti-inflammatory and antioxidant properties, may benefit people with cognitive impairment caused by AD. Choto-san, an herbal mixture with 11 medicinal plants, has been used to treat dementia, improving memory and learning. Kami-untan-to, a Japanese herb, has been found to improve nerve growth in rat brain cells, potentially slowing AD [61]
1.11 Some Novel Targets for Alzheimer’s disease treatment:
Tau aggregation inhibitors:
Antibodies prevent abnormal tau protein aggregation into tangles, aiming to stabilize its normal functional form.
Kinase inhibitors:
Tau phosphorylation is involved in its abnormal aggregation, and inhibiting specific kinases involved in tau phosphorylation can prevent tau pathology and cognitive decline.
Anti-inflammatory approaches:
Inflammation is believed to contribute to AD progression, and targeting neuroinflammation with anti-inflammatory drugs or immune-modulating therapies may help reduce tau pathology.
Apolipoprotein E (APOE):
Certain variants of the APOE gene are linked to an increased risk of Alzheimer's.
Tau degradation enhancers:
The brain's ability to clear abnormal tau is enhanced by boosting the activity of cellular degradation pathways like autophagy or the ubiquitin-proteasome system.
Epigenetic modifications:
Modulating epigenetic processes to regulate gene expression and potentially reverse or slow down Alzheimer's progression.
Microglia activation:
Modulating the activation and function of microglia, the brain's immune cells, to maintain a healthy brain environment.
1.12. How screening methods are useful to screen test drugs
Screening methods help researchers efficiently test numerous compounds for desired pharmacological activity, identifying active compounds, assessing potency and selectivity, evaluating drug-drug interactions, assessing safety and toxicity profiles, and optimizing lead compounds. They measure drug potency by comparing it to the target or disease model, ensuring the drug interacts specifically with the intended target. High-throughput screening (HTS) methods expedite drug discovery by screening thousands to millions of compounds, often using robotic systems and automation to enhance efficiency and reduce human error.
1.13 Brief Information on the overall Principle behind the screening models:
Drug screening models are used by researchers to replicate Alzheimer's disease pathology using genetically modified animal models or cultured cells. They identify molecular targets associated with Alzheimer's disease and test their ability to modulate these targets using existing drugs, natural products, or synthesized molecules. These models evaluate the efficacy of potential Alzheimer's drugs by measuring their impact on disease-related markers and assessing safety and pharmacokinetic properties. Promising compounds identified through screening models can progress to preclinical studies and clinical trials, providing valuable insights into therapeutic effects and safety considerations.
2.1. Inhibition of Acetylcholine-esterase activity in rat striatum
2.1.1.PURPOSE AND RATIONALE:-
This assay screens drugs for acetylcholine-esterase inhibition, potentially useful for Alzheimer's disease treatment, as AChE is responsible for rapid acetylcholine hydrolysis and inactivation. [62]
2.1.2.PROCEDURE:-
Reagents:-
The process involves preparing a phosphate buffer, a substrate, a DTNB, and a stock solution of the test drug, which are serially diluted to a final concentration of 10-4 M. [62]
Tissue Preparation:-
Male Wistar rats are decapitated, brains removed, dissected, and homogenized. A suspension is added to vehicle or test drug, and re-incubated at 37°C. [62]
Assay:-
The Beckman DU-50 spectrophotometer measures enzyme activity, and reagents are added to blank and sample cuvettes & also ,Blank: 0.8ml PO4 buffer/DTNB 0.8ml buffer/Substrate ,Control: 0.8ml PO4 buffer/DTNB/Enzyme 0.8ml PO4 buffer/Substrate ,Drug: 0.8ml PO4 buffer/DTNB/Drug/Enzyme 0.8ml PO4 buffer/Substrate [62]
2.1.3. EVALUATION:-
The substrate concentration is 10 mM diluted 1:2 in an assay to produce a final concentration of 5 mM, which is used for IC50 calculations. The concentration of DTNB is 0.05 mM, resulting in a final concentration of 0.05 mM.
• % Inhibition=slope control−slope drug ×100 slope control,• IC50 values are calculated from log-probit analysis. [62]
Human Serum
2.2.1. PURPOSE AND RATIONALE:-
The substrate concentration is 10 mM diluted 1:2 in an assay to produce a final concentration of 5 mM, which is used for IC50 calculations. The concentration of DTNB is 0.05 mM, resulting in a final concentration of 0.05 mM.. [62]
2.2.2.PROCEDURE:-
Reagents
Add 0.05M phosphate buffer, pH7.2, 6.85g NaH4PO4·H2O/100ml distilled H2O, and 13.40g NaH4PO4·H2O/100ml distilled H2O until pH reaches 7.2. Substrate in buffer (225.8mg s-butyrylthiocholine chloride) q.s. to 100ml with 0.05M phosphate buffer, pH7.2.The drug is serially diluted with 0.5mM DTNB in a buffer, followed by a 2mM stock solution of the test drug in a solvent. [62]
Enzyme Preparation:-
Lyophilized human serum is reconstituted in distilled water, added to test drug, and pre-incubated at 37°C for 10 minutes.
Assay:-
Enzyme activity is measured with the Beckman DU50 spectrophotometer.Reagents are added to the blank and sample cuvettes: Blank: 0.8ml PO4 buffer/DTNB 0.8ml buffer/Substrate.Control: 0.8ml PO4 buffer/DTNB/Enzyme 0.8ml PO4 buffer/Substrate.Drug: 0.8ml PO4 buffer/DTNB/Drug/Enzyme 0.8ml PO4 buffer/Substrate 26 [62]
2.2.3 EVALUATION:-
For IC50 determinations:- Substrate concentration is 10mM diluted 1:2 in assay yielding final concentration of 5mM. DTNB concentration is 0.5mM yielding 0.25mM final concentration[62]
3.% Inhibition= slope control−slope drug × 100 slope control,,IC50 values are calculated from log-probit analysis
2.3. Stimulation of Phosphatidylinositol Turnover in Rat Brain Slices
2.3.1.Principle:
Phosphatidylinositol turnover in rat brain slices is a biochemical assay assessing signaling pathways, measuring inositol phosphate production as a reflection of PI turnover. [62]
2.3.2. Procedure:
Rat brain slices are prepared by dissecting and slicing it into thin sections using a specialized tissue slicer or microtome, then incubated in a suitable buffer to maintain viability. Brain slices are stimulated with neurotransmitters, hormones, or pharmacological agents to activate the phosphoinositide signaling pathway, thereby affecting the turnover of PI enzymes.Brain slices are stimulated, incubated for PI turnover, and harvested for lipid extraction, including PI and its metabolites.The extracted lipids are analyzed using HPLC or radioisotope-based assays to measure IP levels, which indicate PI turnover and the activity of the phosphoinositide signalling pathway. [62]
2.3.3. Evaluation:
The study uses inositol phosphates (IP) levels in brain slices to understand phosphoinositide turnover and signaling pathway activation. It uses dose-response analysis to determine sensitivity and potency, and time-course analysis to identify peak activity or sustained responses. The study suggests that adding specific inhibitors or activators of key enzymes or receptors can help investigate the signaling pathway's mechanisms. [62]
Reagents
Add 0.05M phosphate buffer, pH7.2, 6.85g NaH4PO4·H2O/100ml distilled H2O, and 13.40g NaH4PO4·H2O/100ml distilled H2O until pH reaches 7.2. Substrate in buffer (225.8mg s-butyrylthiocholine chloride) q.s. to 100ml with 0.05M phosphate buffer, pH7.2.The drug is serially diluted with 0.5mM DTNB in a buffer, followed by a 2mM stock solution of the test drug in a solvent. [62]
Enzyme Preparation:-
Lyophilized human serum is reconstituted in distilled water, added to test drug, and pre-incubated at 37°C for 10 minutes.
Assay:-
Enzyme activity is measured with the Beckman DU50 spectrophotometer.Reagents are added to the blank and sample cuvettes: Blank: 0.8ml PO4 buffer/DTNB 0.8ml buffer/Substrate.Control: 0.8ml PO4 buffer/DTNB/Enzyme 0.8ml PO4 buffer/Substrate.Drug: 0.8ml PO4 buffer/DTNB/Drug/Enzyme 0.8ml PO4 buffer/Substrate 26 [62]
2.2.3 EVALUATION:-
For IC50 determinations:- Substrate concentration is 10mM diluted 1:2 in assay yielding final concentration of 5mM. DTNB concentration is 0.5mM yielding 0.25mM final concentration[62]
3.% Inhibition= slope control−slope drug × 100 slope control,,IC50 values are calculated from log-probit analysis
2.3. Stimulation of Phosphatidylinositol Turnover in Rat Brain Slices
2.3.1.Principle:
Phosphatidylinositol turnover in rat brain slices is a biochemical assay assessing signaling pathways, measuring inositol phosphate production as a reflection of PI turnover. [62]
2.3.2. Procedure:
Rat brain slices are prepared by dissecting and slicing it into thin sections using a specialized tissue slicer or microtome, then incubated in a suitable buffer to maintain viability. Brain slices are stimulated with neurotransmitters, hormones, or pharmacological agents to activate the phosphoinositide signaling pathway, thereby affecting the turnover of PI enzymes.Brain slices are stimulated, incubated for PI turnover, and harvested for lipid extraction, including PI and its metabolites.The extracted lipids are analyzed using HPLC or radioisotope-based assays to measure IP levels, which indicate PI turnover and the activity of the phosphoinositide signalling pathway. [62]
2.3.3. Evaluation:
The study uses inositol phosphates (IP) levels in brain slices to understand phosphoinositide turnover and signaling pathway activation. It uses dose-response analysis to determine sensitivity and potency, and time-course analysis to identify peak activity or sustained responses. The study suggests that adding specific inhibitors or activators of key enzymes or receptors can help investigate the signaling pathway's mechanisms. [62]
3.IN-Vivo Screening Models:
3.1. Step-down method:
3.1.1. Purpose and Rationale:
An animal, such as a mouse or rat, often explores a rectangular compartment by stepping down to the floor when placed on an elevated platform.[62]
3.1.2. Procedure:
Requirements: A) Either sex of mice or rats are employed. B) Over the block, a rectangular box (50 by 50) with an electrifiable grid floor 35 fits. C) The shock device and grid floor are linked. [62]
A typical paradigm consists of : a) Familiarization b) Learning c) Retension test
A)Familiarization:
Animal is positioned on the platform. after the cylinder is raised, released Measured is the latency to descend It is brought back to the home cage after 10 seconds of expolaration. [62]
B) Learning:
The animal has immediately lowered itself from the platform. An unnecessary shock is given (foot shock: 50Hz: 1.5 mA; 1 sec). The creature goes back to its own prison. [62]
C) Retension test:
One day following the educational journey Step-down delay is measured once the animal is positioned back on the platform. Once the animal descends or stays on the platform (60 seconds is the cut-off time), the test is considered completed. [62]
3.1.3. Evaluation:
Measured are the descent times during the learning phase and the retention test. Learning is characterised as an extension of the step-down latency.. [62]
3.2. Step-through Method
3.2.1. Purpose and Rationale:-
This test uses the normal behavior of mice and rats. These animals avoid bright light and prefer dim lighting. When placed in a brightly lit space connected to a dark enclosure, they rapidly enter the dark compartment and remain there.[62]
3.2.2. Procedure:-
The test apparatus consists of a small room connected to a large dark room through a guillotine door. The small compartment is illuminated by a 7 W/12 V bulb. In the acquisition test, animals are placed in the illuminated compartment at the maximum distance from the guillotine door and the latency to enter the dark compartment is measured. Animals that do not pass through the door within the cutoff time of 90 seconds (mice) or 180 seconds (rats) are not used. Immediately after the animal enters the darkroom, the door is automatically closed and the inevitable foot shock is administered. The animals are then promptly removed from the apparatus (within 10 seconds) and returned to their home cages. The test procedure is repeated with or without drug; the cutoff time on day 2 is 300 seconds (mice) or 600 seconds (rats), respectively. [62]
3.2.3. Evaluation:-
The study measures step-through time during the learning phase and retention test, focusing on prolonging step-through latencies specific to the experimental situation, and defining learning as an increase in step-through latency. [62]
3.3. Scopolamine –Induced Amnesia In Mice
3.3.1. Purpose and Rationale:
The muscarinic inhibitor scopolamine causes transient amnesia in young volunteers and mice, and several cholinergic drug agonists have been found to reverse this amnesia.[63]
3.3.2. PROCEDURE:
The scopolamine test is conducted on bunches of 10 male NMRI mice weighing between 26-32 g in a single trial. After regulating 3mg/kg of scopolamine hydrobromide through intraperitoneal infusion, each mouse is separately set within the brightly lit segment of a two-chambered device. Taking after a brief introduction period, the mouse enters the moment, darker chamber. Once interior the moment chamber, the entryways are closed to avoid elude. A 1 mA, 1-second foot stun is at that point connected through the network floor. The mouse is at that point returned to its domestic cage. After 24 hours, the testing is rehashed by placing the creature back within the brightly lit chamber. The idleness, or time taken, for the mouse to enter the dim chamber inside a 5-minute test session is measured electronically. In contrast, untreated control creatures enter the darker chamber within the moment trial with a idleness of around 250 seconds. Treatment with scopolamine diminishes the idleness to 50 seconds. Test compounds are managed 90 minutes some time recently the preparing session. The drawn out inactivity shows that the creature recalls being rebuffed and, as a result, maintains a strategic distance from the darker chamber...[63]
3.3.3. Evaluation:
The areas of the test drugs were measured after administration of various doses, with some drugs showing linear dose-response curves and others showing a U-shaped response.[63]
3.4. Ischemia Induced Alzheimer Disease Screening Model
3.4.1 Principle:
Research models of ischemia-induced Alzheimer's disease study brain ischemia and its impact on development and progression by modeling ischemic conditions and assessing cognitive impairment and pathological changes. [63]
3.4.2. Procedure:
Cerebral ischemia in rats using methods such as bilateral carotid artery occlusion, middle cerebral artery occlusion, or global cerebral ischemia reduces blood flow and releases oxygen. The duration and severity of ischemic stroke are determined by careful management and monitoring. Methods such as cerebral blood flow measurements, EEG monitoring or histological analysis are used to ensure homogeneity between the experimental groups. Cognitive function was evaluated by postmortem behavioral tests, including memory, learning, attention, and object recognition, comparing animals with the number 039. Pathological diagnosis uses methods such as immunohistochemistry, ELISA analysis, and Western blotting to evaluate the pathological changes of Alzheimer's disease, including amyloid beta plaques, tau protein chains, neuroinflammation, synapse loss, and neuronal damage. Ischemia-induced Alzheimer's disease models can assess the efficacy of therapeutic interventions targeting specific aspects of the disease, such as beta-amyloid aggregation, tau phosphorylation, or neuroinflammation... [63]
3.4.3 Evaluation:
In this study, we evaluate a diagnostic model of ischemia-induced Alzheimer's disease using animal behavioral tests, pathological changes, neurochemical and molecular analyses, and evaluate interventions in medicine. By comparing cognitive impairment and pathological changes in ischemic and control groups, we will assess the consistency and effectiveness of medical interventions and determine their therapeutic value [63]
The review article emphasizes the types, etiology , neuropathology, mechanism of Alzheimer’s disease, importance of screening of new therapeutic component for this disease, details procedures & significances of in-vivo and in-vitro screening models in evaluating Alzheimer's disease drugs .Also neuropthology,, mechanism of this disease are described here with proper figures. Besides these the In-vivo models, provide a comprehensive approach to studying drug effects in living organisms, while in-vitro models, like cellular and biochemical assays, offer insights into molecular and cellular mechanisms. However, these models may not fully represent the complexity of Alzheimer's disease or the in vivo environment. Combining these models is crucial for drug development.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.

Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.
